PALB2 interacts with KEAP1 to promote NRF2 nuclear accumulation and function - PubMed (original) (raw)
. 2012 Apr;32(8):1506-17.
doi: 10.1128/MCB.06271-11. Epub 2012 Feb 13.
Hong Cai, Tongde Wu, Bijan Sobhian, Yanying Huo, Allen Alcivar, Monal Mehta, Ka Lung Cheung, Shridar Ganesan, Ah-Ng Tony Kong, Donna D Zhang, Bing Xia
Affiliations
- PMID: 22331464
- PMCID: PMC3318596
- DOI: 10.1128/MCB.06271-11
PALB2 interacts with KEAP1 to promote NRF2 nuclear accumulation and function
Jianglin Ma et al. Mol Cell Biol. 2012 Apr.
Abstract
PALB2/FANCN is mutated in breast and pancreatic cancers and Fanconi anemia (FA). It controls the intranuclear localization, stability, and DNA repair function of BRCA2 and links BRCA1 and BRCA2 in DNA homologous recombination repair and breast cancer suppression. Here, we show that PALB2 directly interacts with KEAP1, an oxidative stress sensor that binds and represses the master antioxidant transcription factor NRF2. PALB2 shares with NRF2 a highly conserved ETGE-type KEAP1 binding motif and can effectively compete with NRF2 for KEAP1 binding. PALB2 promotes NRF2 accumulation and function in the nucleus and lowers the cellular reactive oxygen species (ROS) level. In addition, PALB2 also regulates the rate of NRF2 export from the nucleus following induction. Our findings identify PALB2 as a regulator of cellular redox homeostasis and provide a new link between oxidative stress and the development of cancer and FA.
Figures
Fig 1
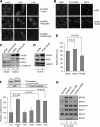
PALB2 interacts with KEAP1. (A) Domain structure and known binding partners of PALB2. (B) Tandem affinity purification of PALB2 from a HeLa S3 cell line stably expressing a FLAG-HA-double-tagged PALB2 protein (PALB2-FH). Numbers at left are molecular masses in kilodaltons. (C) Reciprocal co-IP of endogenous PALB2 and KEAP1 in U2OS cells. (D) Endogenous KEAP1 association with PALB2 and NRF2 under normal and stress conditions. U2OS cells were treated with DMSO (mock), H2O2, tBHQ, and CPT at the indicated concentrations for 2 h, and the interactions were analyzed by IP-Western blotting.
Fig 2
PALB2 directly interacts with the KC domain of KEAP1. (A) Schematic of the KEAP1 constructs. (B) In vitro translations were performed to produce HA-tagged KEAP1 proteins (lanes 1 to 4) and FLAG-HA-PALB2 (lane 5). Subsequently, each of the KEAP1 species was mixed with FLAG-HA-PALB2 and an anti-PALB2 antibody was used to precipitate PALB2. Then, the precipitated materials were analyzed by Western blotting using an anti-HA antibody. (C) Various KEAP1 constructs shown in panel A were transfected into 293T cells, and proteins were IPed by anti-FLAG M2 agarose. The precipitates were analyzed by Western blotting.
Fig 3
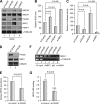
PALB2 binds KEAP1 through a highly conserved ETGE motif. (A) Schematic of PALB2 constructs used in the domain mapping study. (B and C) PALB2 constructs were transiently expressed in 293T cells, proteins were IPed with anti-FLAG M2 agarose, and the precipitates were analyzed by Western blotting. (D) Amino acid sequence alignment of the N-terminal sequences of PALB2 and NRF2 showing the shared LDEETGE KEAP1 binding motif. (E) Deletion or mutations of the ETGE motif in PALB2 all abolish KEAP1 binding. (F) E91R and T92E mutations do not affect the binding of PALB2 to BRCA2, RAD51, and BRCA1.
Fig 4
PALB2 overexpression promotes NRF2 nuclear accumulation and reduces ROS level. (A) An HA-NRF2 plasmid was cotransfected into 293T cells with empty Myc-GFP vector or Myc-PALB2WT-GFP or Myc-PALB2T92E-GFP constructs for 24 h, and their expression and localization were analyzed by fluorescence microscopy. (B) T98G cells overexpressing N-terminally FLAG-HA-double-tagged PALB2 were costained with HA (for PALB2) and NRF2 antibodies and visualized by fluorescence microscopy. (C) T98G cells in panel B were fractionated into cytosol and nuclei, and the amounts of indicated proteins in each compartment were analyzed by Western blotting. (D) Nuclei of HeLa cells harboring the vector or PALB2 overexpression construct were isolated, and protein amounts were analyzed by Western blotting. Lamin A/C, a nuclear protein, was used as a loading control. (E) ROS levels in naïve HeLa cells and the two stable cell lines in panel D were measured using the DCF assay. (F) U2OS cells were cotransfected with the ARE-Luc reporter system and various PALB2 expression vectors, and luciferase activities were measured 48 h after transfection. (G) Cells were transfected with the indicated expression vectors for 30 h, and the tagged PALB2 proteins were IPed with anti-FLAG M2 beads. The IPed PALB2 and co-IPed BRCA2, BRCA1, and KEAP1 proteins were probed with their respective antibodies by Western blotting.
Fig 5
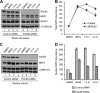
Competition between PALB2 and NRF2 for KEAP1 binding. (A) Disruption of preformed NRF2/KEAP1 complex in cell extracts. Increasing amounts of whole-cell extracts from 293T cells transfected with pOZC1-PALB2WT or pOZC1-PALB2T92E (lanes 2 and 6, respectively) were added to equal aliquots of extract made from cells cotransfected with HA-NRF2 and Myc-KEAP-GFP. Then, KEAP1 was precipitated using a GFP antibody and the amounts of proteins were analyzed by Western blotting. (B and C) Competitive disruption of isolated NRF2/KEAP1 or PALB2/KEAP1 complexes by IVTed PALB2T551 or NRF2, respectively. See Materials and Methods for detailed procedures. (D) In vitro competition between PALB2 and NRF2 for KEAP1 binding. Different amounts of PALB2 and NRF2 were generated together with a fixed amount of KEAP1 in triple IVT reactions by using different combinations of amounts (in μl) of PCR-generated templates for KEAP1, PALB2, and NRF2 (top panels). Then, KEAP1 was IPed and the amounts of KEAP1, PALB2, and NRF2 in the precipitates were analyzed by Western blotting using anti-HA antibody, which recognizes all 3 proteins (bottom panels).
Fig 6
siRNA-mediated depletion of PALB2 reduces NRF2 abundance and activity in the nucleus and increases cellular ROS level. (A) Western blotting showing the levels of proteins of interest after siRNA treatments. (B) ROS levels in cells depleted of each of the indicated proteins. (C) Endogenous NRF2 activity on an ARE-luciferase reporter following depletion of PALB2 and other proteins. (D and E) Nuclear NRF2 level after depletion of PALB2 in U2OS cells analyzed by Western blotting (D) and quantified by densitometry (E). (F and G) ChIP analysis of endogenous NRF2 binding to NQO1 ARE in control and PALB2-depleted cells. (F) An agarose gel image of the PCR products in a representative experiment. (G) Results quantified by real-time PCR.
Fig 7
PALB2 regulates NRF2 nuclear export after induction. (A) Western blotting of whole-cell extracts of U2OS cells (transfected with control or PALB2 siRNAs for 48 h) following treatment with DMSO or tBHQ and at indicated time points after tBHQ removal. (B) Results from 3 independent experiments in panel A were quantified by densitometry and plotted. (C) Western blotting of nuclear extracts of U2OS cells following the same treatments as those in panel A. (D) Results from 3 independent experiments in panel C were quantified by densitometry.
Fig 8
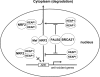
A proposed model of PALB2 regulation of NRF2 in the nucleus. Nuclear NRF2 forms heterodimers with small Maf proteins and activates antioxidant gene expression through association with the AREs in the promoter region of the genes. KEAP1 represses NRF2 function in the nucleus by blocking NRF2 binding to AREs and exporting it to the cytoplasm for degradation. By competitively binding to KEAP1, PALB2 alleviates the negative impact of KEAP1 on NRF2 function via the two above-noted mechanisms. BRCA2 is also a component of the PALB2-KEAP1 complex, but its role is unclear.
Similar articles
- The oncoprotein HBXIP competitively binds KEAP1 to activate NRF2 and enhance breast cancer cell growth and metastasis.
Zhou XL, Zhu CY, Wu ZG, Guo X, Zou W. Zhou XL, et al. Oncogene. 2019 May;38(21):4028-4046. doi: 10.1038/s41388-019-0698-5. Epub 2019 Jan 28. Oncogene. 2019. PMID: 30692632 - A mechanism for the suppression of homologous recombination in G1 cells.
Orthwein A, Noordermeer SM, Wilson MD, Landry S, Enchev RI, Sherker A, Munro M, Pinder J, Salsman J, Dellaire G, Xia B, Peter M, Durocher D. Orthwein A, et al. Nature. 2015 Dec 17;528(7582):422-6. doi: 10.1038/nature16142. Epub 2015 Dec 9. Nature. 2015. PMID: 26649820 Free PMC article. Retracted. - Expression of xCT and activity of system xc(-) are regulated by NRF2 in human breast cancer cells in response to oxidative stress.
Habib E, Linher-Melville K, Lin HX, Singh G. Habib E, et al. Redox Biol. 2015 Aug;5:33-42. doi: 10.1016/j.redox.2015.03.003. Epub 2015 Mar 18. Redox Biol. 2015. PMID: 25827424 Free PMC article. - PALB2: the hub of a network of tumor suppressors involved in DNA damage responses.
Park JY, Zhang F, Andreassen PR. Park JY, et al. Biochim Biophys Acta. 2014 Aug;1846(1):263-75. doi: 10.1016/j.bbcan.2014.06.003. Epub 2014 Jul 3. Biochim Biophys Acta. 2014. PMID: 24998779 Free PMC article. Review. - PALB2/FANCN: recombining cancer and Fanconi anemia.
Tischkowitz M, Xia B. Tischkowitz M, et al. Cancer Res. 2010 Oct 1;70(19):7353-9. doi: 10.1158/0008-5472.CAN-10-1012. Epub 2010 Sep 21. Cancer Res. 2010. PMID: 20858716 Free PMC article. Review.
Cited by
- Comparative analysis of NRF2-responsive gene expression in AcPC-1 pancreatic cancer cell line.
Yi YW, Oh S. Yi YW, et al. Genes Genomics. 2015;37(1):97-109. doi: 10.1007/s13258-014-0253-2. Epub 2014 Dec 5. Genes Genomics. 2015. PMID: 25540678 Free PMC article. - Transgenic Expression of Nrf2 Induces a Pro-Reductive Stress and Adaptive Cardiac Remodeling in the Mouse.
Jyothidasan A, Sunny S, Murugesan S, Quiles JM, Challa AK, Dalley B, Cinghu SK, Nanda V, Rajasekaran NS. Jyothidasan A, et al. Genes (Basel). 2022 Aug 24;13(9):1514. doi: 10.3390/genes13091514. Genes (Basel). 2022. PMID: 36140682 Free PMC article. - Role of Nrf2 and Autophagy in Acute Lung Injury.
Rojo de la Vega M, Dodson M, Gross C, Mansour HM, Lantz RC, Chapman E, Wang T, Black SM, Garcia JG, Zhang DD. Rojo de la Vega M, et al. Curr Pharmacol Rep. 2016 Apr;2(2):91-101. doi: 10.1007/s40495-016-0053-2. Epub 2016 Feb 6. Curr Pharmacol Rep. 2016. PMID: 27313980 Free PMC article. - Keap1 Cystenine 151 as a Potential Target for Artemisitene-Induced Nrf2 Activation.
Liu S, Xu S, Wei R, Cui Z, Wu X, Wei R, Xie L, Zhou Y, Li W, Chen W. Liu S, et al. Biomed Res Int. 2019 Oct 15;2019:5198138. doi: 10.1155/2019/5198138. eCollection 2019. Biomed Res Int. 2019. PMID: 31737667 Free PMC article. - Hyperactivity of the transcription factor Nrf2 causes metabolic reprogramming in mouse esophagus.
Fu J, Xiong Z, Huang C, Li J, Yang W, Han Y, Paiboonrungruan C, Major MB, Chen KN, Kang X, Chen X. Fu J, et al. J Biol Chem. 2019 Jan 4;294(1):327-340. doi: 10.1074/jbc.RA118.005963. Epub 2018 Nov 8. J Biol Chem. 2019. PMID: 30409900 Free PMC article.
References
- Chen P, et al. 2008. Association of common PALB2 polymorphisms with breast cancer risk: a case-control study. Clin. Cancer Res. 14: 5931– 5937 - PubMed
- Erkko H, et al. 2007. A recurrent mutation in PALB2 in Finnish cancer families. Nature 446: 316– 319 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA072720/CA/NCI NIH HHS/United States
- R01ES015010/ES/NIEHS NIH HHS/United States
- R01CA138804/CA/NCI NIH HHS/United States
- R01 ES015010/ES/NIEHS NIH HHS/United States
- R01 CA138804/CA/NCI NIH HHS/United States
- P30 ES005022/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous