Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney - PubMed (original) (raw)
Review
Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney
Volker Vallon et al. Annu Rev Physiol. 2012.
Abstract
Diabetes mellitus affects the kidney in stages. At the onset of diabetes mellitus, in a subset of diabetic patients the kidneys grow large, and glomerular filtration rate (GFR) becomes supranormal, which are risk factors for developing diabetic nephropathy later in life. This review outlines a pathophysiological concept that focuses on the tubular system to explain these changes. The concept includes the tubular hypothesis of glomerular filtration, which states that early tubular growth and sodium-glucose cotransport enhance proximal tubule reabsorption and make the GFR supranormal through the physiology of tubuloglomerular feedback. The diabetic milieu triggers early tubular cell proliferation, but the induction of TGF-β and cyclin-dependent kinase inhibitors causes a cell cycle arrest and a switch to tubular hypertrophy and a senescence-like phenotype. Although this growth phenotype explains unusual responses like the salt paradox of the early diabetic kidney, the activated molecular pathways may set the stage for tubulointerstitial injury and diabetic nephropathy.
Figures
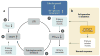
Figure 1
Tubular hypothesis of glomerular filtration in diabetes mellitus. (a) Glomerular hyperfiltration in diabetes mellitus as a primary tubular event. The single-nephron glomerular filtration rate (SNGFR) is determined by primary vascular events, tubuloglomerular feedback, and tubuloglomerular feedback resetting. Glomerulotubular balance (GTB) and primary tubular events determine the concentration and delivery of Na+, Cl−, and K+ at the luminal macula densa (MDNaClK). A primary vascular event causes SNGFR and MDNaClK to change in the same direction, whereas a primary tubular event causes SNGFR and MDNaClK to change in opposite directions. Hyperglycemia causes a primary increase in proximal tubule reabsorption (the primary tubular event) through enhanced tubular growth and Na+-glucose cotransport➊. This reduces MDNaClK ➋ and, via tubuloglomerular feedback➌, increases SNGFR➍. Enhanced growth and tubular reabsorption can also reduce the hydrostatic pressure in Bowman space (_P_BOW) ➎, which by increasing effective filtration pressure can also increase SNGFR➍. The resulting increase in SNGFR partly restores the fluid and electrolyte load to the distal nephron. (b) The salt paradox. The nondiabetic kidney adjusts NaCl transport to dietary NaCl intake primarily downstream of the macula densa, and thus MDNaClK or SNGFR is not altered. In contrast, diabetes renders reabsorption in the proximal tubule very sensitive to dietary NaCl, with subsequent effects on MDNaClK and SNGFR.
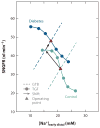
Figure 2
A proposed role for tubuloglomerular feedback (TGF) resetting in the diabetic kidney. TGF is depicted as the inverse relationship between early distal Na+ concentration ([Na+]early distal) and single-nephron GFR (SNGFR). Data from perturbation analysis of late proximal tubule flow rate were combined with data for fractional proximal reabsorption (29), ambient [Na+]early distal (30), and the relationship between late proximal flow rate and early distal conductivity (29) in control and STZ-diabetic rats. Similar curves were derived for early distal Cl− concentration (not shown). Dashed lines represent glomerular-tubular balance (GTB), which refers to the load dependency of reabsorption between the glomerulus and the macula densa. The operating point (triangles) of the nephron, where TGF and GTB intersect, is usually located where TGF is steep. A primary diabetes--induced increase in reabsorption (with a parallel shift in the GTB line) lowers [Na+]early distal, which increases SNGFR but shifts the operating point to the flatter part of the TGF curve. To operate at high TGF efficiency, the TGF curve in diabetes resets upward such that the operating point is restored to the steeper part of the TGF curve.
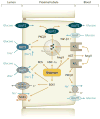
Figure 3
Proximal tubule glucose and Na+ transport in the diabetic kidney. Hyperglycemia increases glomerular filtration of glucose and enhances glucose reabsorption in the proximal tubule via SGLT2 (early segments) and SGLT1 (later segments), thereby enhancing the reabsorption and intracellular concentration of Na+ ([Na+]i). An increase in [Na+]i and activation of basolateral Na-K-ATPase [via diabetes-induced protein kinase C β1 (PKCβ1)] have been implicated in diabetic tubular growth. Diabetes increases angiotensin II (Ang II) in the cytosol and in the extracellular space; the latter may include enhanced release of angiotensinogen (AGT) (75). Release of AGT and activation of AT1 receptors may also occur at the luminal membrane. Ang II and hepatocyte nuclear factor HNF-1α increase SGLT2 and glucose transporter (GLUT)2 expression. Diabetes also induces the serum- and glucocorticoid-inducible kinase SGK1, which increases SGLT1 activity. Induction of oxidative stress (ROS) can inhibit SGLT. Glucose reabsorption via SGLTs is electrogenic, and luminal K+ channels (e.g., SGK1-activated KCNE1/KCNQ1 in the late proximal tubule) stabilize the membrane potential. Glucose exits via basolateral GLUTs. In diabetes, PKCβ1 can shift part of GLUT2 into the apical membrane, which would equilibrate luminal and basolateral glucose concentrations. The induction of transforming growth factor β1 (TGF-β1) may be particularly sensitive to basolateral glucose uptake. ROS denotes reactive oxygen species. Modified with permission from Reference .
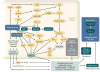
Figure 4
The early growth phenotype of the diabetic proximal tubule and potential links to renal injury and failure. Diabetes mellitus--induced growth of the proximal tubule includes an initial phase of cell proliferation and an early transition to hypertrophy via G1 cell cycle arrest and the development of a senescence-like phenotype. The molecular pathways involved in this tubular growth phenotype are linked to tubulointerstitial fibrosis and inflammation and may thus set the stage for renal failure later in life. Abbreviations: AGE, advanced glycation end products; Ang II, angiotensin II; bFGF, basic fibroblast growth factor; CTGF, connective tissue growth factor; Cyc D1, cyclin D1; HGF, hepatocyte growth factor; HIF-1α, hypoxia-inducible factor 1α; IGF-1, insulin-like growth factor 1; PKCβ1, protein kinase C β1; JAK/STAT, Janus kinase/signal transducers and activators of transcription; MCP-1, monocyte chemotactic protein-1; mTORC1, mammalian target of rapamycin complex 1; ODC, ornithine decarboxylase; p16, p16INK4A; p21, the CDK inhibitor waf1/cip1; p27, p27KIP1; pAMPK, phosphorylated AMP-activated protein kinase; PI3K, phosphoinositide 3-kinase; PDGF-β, platelet-derived growth factor β; RAGE, receptor for AGE; RAS, renin-angiotensin system; ROS, reactive oxygen species; SOCS, suppressor of cytokine signaling; TGF-β, transforming growth factor β; TSC, tuberous sclerosis complex; VEGF, vascular endothelial growth factor. Modified, with permission, from Reference .
Similar articles
- The tubular hypothesis of nephron filtration and diabetic kidney disease.
Vallon V, Thomson SC. Vallon V, et al. Nat Rev Nephrol. 2020 Jun;16(6):317-336. doi: 10.1038/s41581-020-0256-y. Epub 2020 Mar 9. Nat Rev Nephrol. 2020. PMID: 32152499 Free PMC article. Review. - Sodium/glucose cotransporter 2 inhibitors and prevention of diabetic nephropathy: targeting the renal tubule in diabetes.
De Nicola L, Gabbai FB, Liberti ME, Sagliocca A, Conte G, Minutolo R. De Nicola L, et al. Am J Kidney Dis. 2014 Jul;64(1):16-24. doi: 10.1053/j.ajkd.2014.02.010. Epub 2014 Mar 25. Am J Kidney Dis. 2014. PMID: 24673844 Review. - Glomerular and tubular function in the diabetic kidney.
Blantz RC, Singh P. Blantz RC, et al. Adv Chronic Kidney Dis. 2014 May;21(3):297-303. doi: 10.1053/j.ackd.2014.03.006. Adv Chronic Kidney Dis. 2014. PMID: 24780458 Review. - Tubular reabsorption and diabetes-induced glomerular hyperfiltration.
Persson P, Hansell P, Palm F. Persson P, et al. Acta Physiol (Oxf). 2010 Sep;200(1):3-10. doi: 10.1111/j.1748-1716.2010.02147.x. Epub 2010 May 27. Acta Physiol (Oxf). 2010. PMID: 20518753 Free PMC article. Review. - The proximal tubule in the pathophysiology of the diabetic kidney.
Vallon V. Vallon V. Am J Physiol Regul Integr Comp Physiol. 2011 May;300(5):R1009-22. doi: 10.1152/ajpregu.00809.2010. Epub 2011 Jan 12. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21228342 Free PMC article. Review.
Cited by
- Elucidating the complex interplay between chronic kidney disease and hypertension.
Nagata D, Hishida E. Nagata D, et al. Hypertens Res. 2024 Oct 16. doi: 10.1038/s41440-024-01937-8. Online ahead of print. Hypertens Res. 2024. PMID: 39415028 Review. - Renal Sugar Metabolites and mRNA Expression of Glucose Transporters in Meat-Type Chickens with Differing Residual Water Intake.
Milfort MC, Ghareeb AFA, Ariyo OW, Kwakye J, Hartono E, Sovi S, Aryal B, Fuller AL, El Sabry MI, Stino F, Rekaya R, Aggrey SE. Milfort MC, et al. Animals (Basel). 2024 Oct 9;14(19):2912. doi: 10.3390/ani14192912. Animals (Basel). 2024. PMID: 39409861 Free PMC article. - Modern Challenges in Type 2 Diabetes: Balancing New Medications with Multifactorial Care.
Caturano A, Galiero R, Rocco M, Tagliaferri G, Piacevole A, Nilo D, Di Lorenzo G, Sardu C, Vetrano E, Monda M, Marfella R, Rinaldi L, Sasso FC. Caturano A, et al. Biomedicines. 2024 Sep 7;12(9):2039. doi: 10.3390/biomedicines12092039. Biomedicines. 2024. PMID: 39335551 Free PMC article. Review. - Association of Serum Tsukushi Levels with Urinary Albumin-Creatinine Ratio in Type 2 Diabetes Patients.
Li Y, Deng X, Wu X, Zhou L, Yuan G. Li Y, et al. Diabetes Metab Syndr Obes. 2024 Sep 4;17:3295-3303. doi: 10.2147/DMSO.S468228. eCollection 2024. Diabetes Metab Syndr Obes. 2024. PMID: 39252872 Free PMC article. - Proximal tubule hypertrophy and hyperfunction: a novel pathophysiological feature in disease states.
Kanbay M, Copur S, Guldan M, Ozbek L, Hatipoglu A, Covic A, Mallamaci F, Zoccali C. Kanbay M, et al. Clin Kidney J. 2024 Jun 25;17(7):sfae195. doi: 10.1093/ckj/sfae195. eCollection 2024 Jul. Clin Kidney J. 2024. PMID: 39050867 Free PMC article. Review.
References
- Ziyadeh FN, Wolf G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev. 2008;4:39–45. - PubMed
- Jerums G, Premaratne E, Panagiotopoulos S, Macisaac RJ. The clinical significance of hyperfiltration in diabetes. Diabetologia. 2010;53:2093–104. - PubMed
- Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia. 2009;52:691–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK028602/DK/NIDDK NIH HHS/United States
- P30DK079337/DK/NIDDK NIH HHS/United States
- R01 DK056248/DK/NIDDK NIH HHS/United States
- P30 DK079337/DK/NIDDK NIH HHS/United States
- R01 GM066232/GM/NIGMS NIH HHS/United States
- R01 HL094728/HL/NHLBI NIH HHS/United States
- R01GM66232/GM/NIGMS NIH HHS/United States
- R01HL094728/HL/NHLBI NIH HHS/United States
- R01DK28602/DK/NIDDK NIH HHS/United States
- R01DK56248/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical