Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial - PubMed (original) (raw)
Clinical Trial
. 2012 Mar 10;379(9819):895-904.
doi: 10.1016/S0140-6736(12)60195-0. Epub 2012 Feb 14.
Rachel R Smith 1, Ke Cheng 1, Konstantinos Malliaras 1, Louise Ej Thomson 1, Daniel Berman 1, Lawrence Sc Czer 1, Linda Marbán 1, Adam Mendizabal 2, Peter V Johnston 3, Stuart D Russell 3, Karl H Schuleri 3, Albert C Lardo 3, Gary Gerstenblith 3, Eduardo Marbán 4
Affiliations
- PMID: 22336189
- PMCID: PMC4326004
- DOI: 10.1016/S0140-6736(12)60195-0
Clinical Trial
Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial
Raj R Makkar et al. Lancet. 2012.
Abstract
Background: Cardiosphere-derived cells (CDCs) reduce scarring after myocardial infarction, increase viable myocardium, and boost cardiac function in preclinical models. We aimed to assess safety of such an approach in patients with left ventricular dysfunction after myocardial infarction.
Methods: In the prospective, randomised CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction (CADUCEUS) trial, we enrolled patients 2-4 weeks after myocardial infarction (with left ventricular ejection fraction of 25-45%) at two medical centres in the USA. An independent data coordinating centre randomly allocated patients in a 2:1 ratio to receive CDCs or standard care. For patients assigned to receive CDCs, autologous cells grown from endomyocardial biopsy specimens were infused into the infarct-related artery 1·5-3 months after myocardial infarction. The primary endpoint was proportion of patients at 6 months who died due to ventricular tachycardia, ventricular fibrillation, or sudden unexpected death, or had myocardial infarction after cell infusion, new cardiac tumour formation on MRI, or a major adverse cardiac event (MACE; composite of death and hospital admission for heart failure or non-fatal recurrent myocardial infarction). We also assessed preliminary efficacy endpoints on MRI by 6 months. Data analysers were masked to group assignment. This study is registered with ClinicalTrials.gov, NCT00893360.
Findings: Between May 5, 2009, and Dec 16, 2010, we randomly allocated 31 eligible participants of whom 25 were included in a per-protocol analysis (17 to CDC group and eight to standard of care). Mean baseline left ventricular ejection fraction (LVEF) was 39% (SD 12) and scar occupied 24% (10) of left ventricular mass. Biopsy samples yielded prescribed cell doses within 36 days (SD 6). No complications were reported within 24 h of CDC infusion. By 6 months, no patients had died, developed cardiac tumours, or MACE in either group. Four patients (24%) in the CDC group had serious adverse events compared with one control (13%; p=1·00). Compared with controls at 6 months, MRI analysis of patients treated with CDCs showed reductions in scar mass (p=0·001), increases in viable heart mass (p=0·01) and regional contractility (p=0·02), and regional systolic wall thickening (p=0·015). However, changes in end-diastolic volume, end-systolic volume, and LVEF did not differ between groups by 6 months.
Interpretation: We show intracoronary infusion of autologous CDCs after myocardial infarction is safe, warranting the expansion of such therapy to phase 2 study. The unprecedented increases we noted in viable myocardium, which are consistent with therapeutic regeneration, merit further assessment of clinical outcomes.
Funding: US National Heart, Lung and Blood Institute and Cedars-Sinai Board of Governors Heart Stem Cell Center.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
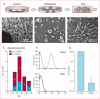
Figure 1. Manufacture and characteristics of CDCs
(A–D) Process flow for manufacture of CDCs. Biopsy specimens are minced into about 1 mm explants that spontaneously yield outgrowth cells (seen budding off the explant in B). These explants are harvested and plated in suspension culture to enable the self-assembly of three-dimensional cardiospheres (C). Subsequent replating of cardiospheres on adherent culture flasks yields CDCs (D). Histogram of time to achievement of the prespecified dose (E). As criteria for identity, representative histograms of flow cytometry data (F) and pooled data (G; logarithmic axis) show that more than 98% of cells expressed CD105, whereas fewer than 0·5% expressed CD45. CDC=cardiosphere-derived cell.
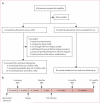
Figure 2. Trial profile and study timeline
(A) CADUCEUS trial profile. (B) Study events and timeline. Major efficacy data are based on comparisons of the baseline MRIs and the 6-month and 12-month MRIs. Study procedures below the timeline apply only to those participants who were randomly allocated to receive CDCs, but all participants underwent the MRI studies shown above the timeline. CDC=cardiosphere-derived cell. *Two patients in the low dose group and four in the high dose group. †Delay due to investigation of contamination.
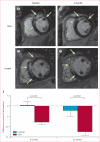
Figure 3. Representative MRI and changes in scar size
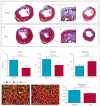
Short-axis MRI of heart at baseline (82 days after myocardial infarction; A) and 6 months after CDC infusion (B) in a participant randomly allocated to receive CDCs. Short-axis MRI of heart at baseline (77 days after myocardial infarction; C) and after 6 months (D) in a control. Infarct scar tissue (green arrows) is evident by areas of hyperintensity (white) whereas viable myocardium appears dark. Difference in scar size from baseline to 6 months (E) or 12 months (F). CDC=cardiosphere-derived cell.
Figure 4. Scar mass and viable left ventricular mass on MRI
We noted decreases in scar mass and increases in viable mass on MRI in patients treated with CDCs but not controls. (A) Differences in scar mass between groups from baseline to 6 months or 12 months. (B) Differences in viable left ventricular mass from baseline to 6 months or 12 months. (C) Correlation between the change in scar mass and the change in viable mass in individual patients at 6 and 12 months compared with baseline. CDC=cardiosphere-derived cell.
Figure 5. Myocardial regeneration in rats treated with CDCs
Representative images of Masson trichrome-stained sections of vehicle control (A) and intracoronary CDC-treated (B) rat hearts 3 weeks after treatment (scar tissue stained blue and viable myocardium stained red). Enlarged regions show striking differences in transmurality of scar and extent of viable myocardium in the infarcted region. (C) Quantification of scar size, scar mass, and viable mass in CDC-treated and control hearts. (D) Cardiomyocyte cross-sectional area in the peri-infarct area of CDC-treated and control hearts shown no hypertrophy in the CDC-treated hearts. CDC=cardiosphere-derived cell. DAPI=4’,6-diamidino-2-phenylindole. α-SA=α-sarcomeric actinin.
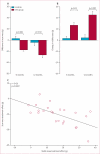
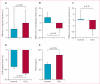
Figure 6. Global function, chamber volumes, and regional function in participants in the CADUCEUS study
(A) Treatment effects (baseline vs 6 months) for MRI-derived ejection fraction. (B) Treatment effects (baseline vs 6 months) for end-diastolic volume. (C) Treatment effects (baseline vs 6 months) for end-systolic volume. (D) Regional strain in infarct-related segments at 6 months. (E) Systolic thickening in infarct-related segments at 6 months. CDC=cardiosphere-derived cell.
Comment in
- Cardiac regeneration: messages from CADUCEUS.
Siu CW, Tse HF. Siu CW, et al. Lancet. 2012 Mar 10;379(9819):870-871. doi: 10.1016/S0140-6736(12)60236-0. Epub 2012 Feb 14. Lancet. 2012. PMID: 22336188 No abstract available. - Stem cells: Myocardial regeneration after infarction-promising phase I trial results.
Lim GB. Lim GB. Nat Rev Cardiol. 2012 Feb 28;9(4):187. doi: 10.1038/nrcardio.2012.25. Nat Rev Cardiol. 2012. PMID: 22371111 No abstract available. - Cardiosphere-derived cells for heart regeneration.
Masuda S, Montserrat N, Okamura D, Suzuki K, Belmonte JCI. Masuda S, et al. Lancet. 2012 Jun 30;379(9835):2425-2426. doi: 10.1016/S0140-6736(12)61062-9. Lancet. 2012. PMID: 22748584 No abstract available. - Cardiosphere-derived cells for heart regeneration.
Fogel J, Znamensky V. Fogel J, et al. Lancet. 2012 Jun 30;379(9835):2426. doi: 10.1016/S0140-6736(12)61063-0. Lancet. 2012. PMID: 22748585 No abstract available.
Similar articles
- Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction).
Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, Marbán L, Mendizabal A, Cingolani E, Johnston PV, Gerstenblith G, Schuleri KH, Lardo AC, Marbán E. Malliaras K, et al. J Am Coll Cardiol. 2014 Jan 21;63(2):110-22. doi: 10.1016/j.jacc.2013.08.724. Epub 2013 Sep 11. J Am Coll Cardiol. 2014. PMID: 24036024 Free PMC article. Clinical Trial. - Intracoronary ALLogeneic heart STem cells to Achieve myocardial Regeneration (ALLSTAR): a randomized, placebo-controlled, double-blinded trial.
Makkar RR, Kereiakes DJ, Aguirre F, Kowalchuk G, Chakravarty T, Malliaras K, Francis GS, Povsic TJ, Schatz R, Traverse JH, Pogoda JM, Smith RR, Marbán L, Ascheim DD, Ostovaneh MR, Lima JAC, DeMaria A, Marbán E, Henry TD. Makkar RR, et al. Eur Heart J. 2020 Sep 21;41(36):3451-3458. doi: 10.1093/eurheartj/ehaa541. Eur Heart J. 2020. PMID: 32749459 Clinical Trial. - Validation of contrast-enhanced magnetic resonance imaging to monitor regenerative efficacy after cell therapy in a porcine model of convalescent myocardial infarction.
Malliaras K, Smith RR, Kanazawa H, Yee K, Seinfeld J, Tseliou E, Dawkins JF, Kreke M, Cheng K, Luthringer D, Ho CS, Blusztajn A, Valle I, Chowdhury S, Makkar RR, Dharmakumar R, Li D, Marbán L, Marbán E. Malliaras K, et al. Circulation. 2013 Dec 24;128(25):2764-75. doi: 10.1161/CIRCULATIONAHA.113.002863. Epub 2013 Sep 23. Circulation. 2013. PMID: 24061088 Free PMC article. - Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a meta-analysis of randomised controlled clinical trials.
Delewi R, Andriessen A, Tijssen JG, Zijlstra F, Piek JJ, Hirsch A. Delewi R, et al. Heart. 2013 Feb;99(4):225-32. doi: 10.1136/heartjnl-2012-302230. Epub 2012 Aug 8. Heart. 2013. PMID: 22875736 Review.
Cited by
- Stem cell therapy for heart failure: Medical breakthrough, or dead end?
Rheault-Henry M, White I, Grover D, Atoui R. Rheault-Henry M, et al. World J Stem Cells. 2021 Apr 26;13(4):236-259. doi: 10.4252/wjsc.v13.i4.236. World J Stem Cells. 2021. PMID: 33959217 Free PMC article. Review. - Cardiac regeneration in children.
Rupp S, Schranz D. Rupp S, et al. Pediatr Cardiol. 2015 Apr;36(4):713-8. doi: 10.1007/s00246-015-1120-x. Epub 2015 Jan 30. Pediatr Cardiol. 2015. PMID: 25633820 Review. - A naturally derived cardiac extracellular matrix enhances cardiac progenitor cell behavior in vitro.
French KM, Boopathy AV, DeQuach JA, Chingozha L, Lu H, Christman KL, Davis ME. French KM, et al. Acta Biomater. 2012 Dec;8(12):4357-64. doi: 10.1016/j.actbio.2012.07.033. Epub 2012 Jul 27. Acta Biomater. 2012. PMID: 22842035 Free PMC article. - Mesenchymal Stem Cells in Cardiology.
White IA, Sanina C, Balkan W, Hare JM. White IA, et al. Methods Mol Biol. 2016;1416:55-87. doi: 10.1007/978-1-4939-3584-0_4. Methods Mol Biol. 2016. PMID: 27236666 Free PMC article. - Persistent diastolic dysfunction in chronically ischemic hearts following coronary artery bypass graft.
Aggarwal R, Qi SS, So SW, Swingen C, Reyes CP, Rose R, Wright C, Hocum Stone LL, Nixon JP, McFalls EO, Butterick TA, Kelly RF. Aggarwal R, et al. J Thorac Cardiovasc Surg. 2023 Jun;165(6):e269-e279. doi: 10.1016/j.jtcvs.2022.08.010. Epub 2022 Aug 24. J Thorac Cardiovasc Surg. 2023. PMID: 36154976 Free PMC article.
References
- Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–65. - PubMed
- de Haan S, Meijers TA, Knaapen P, Beek AM, van Rossum AC, Allaart CP. Scar size and characteristics assessed by CMR predict ventricular arrhythmias in ischaemic cardiomyopathy: comparison of previously validated models. Heart. 2011;97:1951–56. - PubMed
- Orn S, Manhenke C, Anand IS, et al. Effect of left ventricular scar size, location, and transmurality on left ventricular remodeling with healed myocardial infarction. Am J Cardiol. 2007;99:1109–14. - PubMed
- Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–21. - PubMed
- Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–209. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials