Targeting the Anti-Apoptotic Protein c-FLIP for Cancer Therapy - PubMed (original) (raw)
Targeting the Anti-Apoptotic Protein c-FLIP for Cancer Therapy
Ahmad R Safa et al. Cancers (Basel). 2011 Jun.
Abstract
Cellular FLICE (FADD-like IL-1beta-converting enzyme)-inhibitory protein (c-FLIP) is a major resistance factor and critical anti-apoptotic regulator that inhibits tumor necrosis factor-alpha (TNF-alpha), Fas-L, and TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis as well as chemotherapy-triggered apoptosis in malignant cells. c-FLIP is expressed as long (c-FLIP(L)), short (c-FLIP(S)), and c-FLIP(R) splice variants in human cells. c-FLIP binds to FADD and/or caspase-8 or -10 in a ligand-dependent and-independent fashion, which in turn prevents death-inducing signaling complex (DISC) formation and subsequent activation of the caspase cascade. Moreover, c-FLIP(L) and c-FLIP(S) are known to have multifunctional roles in various signaling pathways, as well as activating and/or upregulating several cytoprotective signaling molecules. Upregulation of c-FLIP has been found in various tumor types, and its downregulation has been shown to restore apoptosis triggered by cytokines and various chemotherapeutic agents. Hence, c-FLIP is an important target for cancer therapy. For example, small interfering RNAs (siRNAs) that specifically knockdown the expression of c-FLIP(L) in diverse human cancer cell lines augmented TRAIL-induced DISC recruitment and increased the efficacy of chemotherapeutic agents, thereby enhancing effector caspase stimulation and apoptosis. Moreover, small molecules causing degradation of c-FLIP as well as decreasing mRNA and protein levels of c-FLIP(L) and c-FLIP(S) splice variants have been found, and efforts are underway to develop other c-FLIP-targeted cancer therapies. This review focuses on (1) the functional role of c-FLIP splice variants in preventing apoptosis and inducing cytokine and drug resistance; (2) the molecular mechanisms that regulate c-FLIP expression; and (3) strategies to inhibit c-FLIP expression and function.
Figures
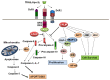
Figure 1.
Schematic overview of the multifunctional roles of c-FLIP in the TRAIL-triggered apoptosis pathway as well as activating various anti-apoptotic and cell survival signaling pathways.
Figure 2.
Structures of c-FLIP isoforms. Three c-FLIP isoforms, c-FLIPL, c-FLIPs, and c-FLIPR, contain two death effector domains (DEDs) at their N-termini. In addition to two DEDs, c-FLIPL contains a large (p20) and a small (p12) caspase-like domain without catalytic activity. c-FLIPS and c-FLIPR consist of two DEDs and a small C-terminus [36].
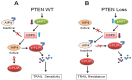
Figure 3.
Schematic model of PTEN-mediated control of c-FLIPS ubiquitination and TRAIL sensitivity. (A). USP8 interacts with AIP4 which can ubiquitinate c-FLIPS leading to its degradation; (B). Increase in pAkt decreases USP8 expression, turns off the USP8/AIP4 ubiquitin switch, resulting in c-FLIPS accumulation. Modified from Panner et al. [114].
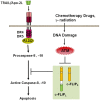
Figure 4.
ATM kinase activity downregulates c-FLIPL and c-FLIPS at the protein level and connects DNA damage signaling to TRAIL-induced apoptosis signaling pathway. DNA damaging agents induce ATM activation, which promotes c-FLIPL protein degradation and c-FLIPS downregulation through an unknown mechanism (Stagni et al. [122]).
References
- Perez E.A. Microtubule inhibitors: Differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol. Cancer Ther. 2009;8:2086–2095. - PubMed
- Clarke R., Leonessa F., Trock B. Multidrug resistance/P-glycoprotein and breast cancer: Review and meta-analysis. Semin. Oncol. 2005;32:S9–S15. - PubMed
- Glavinas H., Krajcsi P., Cserepes J., Sarkadi B. The role of ABC transporters in drug resistance, metabolism and toxicity. Curr. Drug Deliv. 2004;1:27–42. - PubMed
- Roberti A., La Sala D., Cinti C. Multiple genetic and epigenetic interacting mechanisms contribute to clonally selection of drug-resistant tumors: Current views and new therapeutic prospective. J. Cell. Physiol. 2006;207:571–581. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous