Microglial cell activation is a source of metalloproteinase generation during hemorrhagic transformation - PubMed (original) (raw)
Microglial cell activation is a source of metalloproteinase generation during hemorrhagic transformation
Gregory J del Zoppo et al. J Cereb Blood Flow Metab. 2012 May.
Abstract
Hemorrhage and edema accompany evolving brain tissue injury after ischemic stroke. In patients, these events have been associated with metalloproteinase (MMP)-9 in plasma. Both the causes and cellular sources of MMP-9 generation in this setting have not been defined. MMP-2 and MMP-9 in nonhuman primate tissue in regions of plasma leakage, and primary murine microglia and astrocytes, were assayed by immunocytochemistry, zymography, and real-time RT-PCR. Ischemia-related hemorrhage was associated with microglial activation in vivo, and with the leakage of plasma fibronectin and vitronectin into the surrounding tissue. In strict serum-depleted primary cultures, by zymography, pro-MMP-9 was generated by primary murine microglia when exposed to vitronectin and fibronectin. Protease secretion was enhanced by experimental ischemia (oxygen-glucose deprivation, OGD). Primary astrocytes, on each matrix, generated only pro-MMP-2, which decreased during OGD. Microglia-astrocyte contact enhanced pro-MMP-9 generation in a cell density-dependent manner under normoxia and OGD. Compatible with observations in a high quality model of focal cerebral ischemia, microglia, but not astrocytes, respond to vitronectin and fibronectin, found when plasma extravasates into the injured region. Astrocytes alone do not generate pro-MMP-9. These events explain the appearance of MMP-9 antigen in association with ischemia-induced cerebral hemorrhage and edema.
Figures
Figure 1
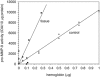
Relative expressions of pro-MMP-9 in striatal tissue with detectable parenchymal hemorrhage versus autologous blood mixed with homogenized normal striatal tissue ex vivo, by zymography (integrated density (IDA), normalized to 10 _μ_g protein). pro-MMP-9 content of tissue with parenchymal hemorrhage (tissue, squares; _R_2=0.60, _2P_=0.015) was significantly greater than the equivalent amount of autologous blood added to homogenates of normal striatal tissue by hemoglobin content (control, triangles; _R_2=0.994, 2P<0.001). The difference between the two relationships is significant (_F_1,19=8.04, _2P_=0.01). See text. MMP, metalloproteinase.
Figure 2
Microglial cells within regions of hemorrhagic transformation of the ischemic core 24 hours after middle cerebral artery occlusion (MCAO) in paraformaldehyde-fixed sections (A). Cells expressing the surface markers CD11b and CD68/KP-1 (B, D, and F and C, E, and G, respectively), compatible with microglial cells or macrophages (arrows), are seen inside (D, E) and outside (F, G) the areas of hemoglobin deposition. Regions of interest outside and inside the areas of hemoglobin deposition are indicated in (B) (that also apply to C). The morphologic features of the identified cells (arrows) suggest activated microglia or macrophages (but not PMN leukocytes) between the two regions of interest in (D) and (E), compared with (F) and (G). Magnification bars: (A–C) 100 _μ_m; (D, E) 10 _μ_m; (F, G) 10 _μ_m.
Figure 3
Plasma matrix protein extravasation in a region of hemorrhagic transformation 2 hours after middle cerebral artery occlusion (MCAO) in paraformaldehyde-fixed sections (A). Hemoglobin deposition identified by gray-blue coloration within the region of injury. Extravasated albumin in tissue surrounding hemorrhage (B), coregisters with the deposition of fibronectin (C) and vitronectin (D) at the hemorrhage boundary. Magnification bar=200 _μ_m (see Supplementary Figure 1).
Figure 4
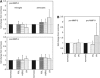
Gelatinase gene transcription responses of primary microglia and astrocytes to matrix exposure under oxygen-glucose deprivation (OGD) compared with normoxia. (A) Responses of microglia and astrocytes to poly-
D
-lysine (PDL), perlecan (HSPG), and laminin (LN). With OGD, microglia displayed no change in MMP-2 or MMP-9 gene expression, while astrocytes displayed increased MMP-2 transcription on LN (all changes not different from unity). _n_=5 to 8 experiments, each in duplicate. (B) Gelatinase gene transcription responses of primary microglia to fibrinogen (FN) and vitronectin (VN) exposed to OGD. VN stimulates a variable increase in MMP-9 gene expression (_2P_=0.15), and fibronectin a consistent decrease in MMP-2 gene expression (_2P_=0.03). _n_=7 experiments, each in duplicate. Bars=mean with standard deviation. MMP, metalloproteinase.
Figure 5
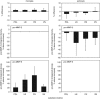
Microglial cell (left panel, _n_=8) and astrocyte (right panel, _n_=5) pro-MMP-2 and pro-MMP-9 secretion in response to matrix protein exposure, under normoxia with fetal bovine serum (FBS) depletion (by zymography). No active MMP-2 or MMP-9 was detected. Matrix substrates include poly-
D
-lysine (PDL), laminin (LN), fibronectin (FN), or vitronectin (VN). Microglial cells produced significant increases in pro-MMP-9 compared with baseline (2P<0.0005 each substrate). VN increased pro-MMP-9 production compared with PDL (_2P_=0.0002), whereas LN decreased pro-MMP-9 compared with PDL (_2P_=0.0148). Astrocytes produced a uniform increase in pro-MMP-2 generation compared with baseline (_2P_=0.0075 each substrate). Bars=mean with standard deviation. MMP, metalloproteinase.
Figure 6
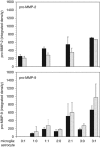
Relative pro-MMP-2 and pro-MMP-9 secretion by primary microglia (left panel, _n_=8) and astrocytes (right panel, _n_=5) to matrix protein exposure, under oxygen-glucose deprivation (OGD) with fetal bovine serum (FBS) depletion (by zymography). Matrix substrates include poly-
D
-lysine (PDL), laminin (LN), fibronectin (FN), or vitronectin (VN). Note the additional increase in pro-MMP-9 production by microglial cells (2P<0.0004 each substrate), but no significant change in pro-MMP-2 production by astrocytes. For microglia, FN stimulated additional pro-MMP-9 production compared with PDL (_2P_=0.0006). The conditions did not appreciably change cell viability during each experiment (upper panels). Bars=mean with standard deviation. MMP, metalloproteinase.
Figure 7
pro-MMP-9 generation depends on relative microglial density and astrocyte exposure. Microglia enhance pro-MMP-9 generation in the presence of astrocytes (grown on collagen IV) under normoxia and oxygen-glucose deprivation (OGD), compared with microglia alone. At a microglia-to-astrocyte ratio of 3:1 pro-MMP-9 secretion significantly increased during OGD compared with normoxia (_F_1,8=12.34, _2P_=0.008), and pro-MMP-9 secretion significantly increased in microglia-to-astrocyte cocultures compared with microglia alone (_F_1,8=6.29, _2P_=0.036). Bars (triplicate, mean with standard deviation): black=normoxia; hatched=OGD. MMP, metalloproteinase.
Similar articles
- Fibronectin- and vitronectin-induced microglial activation and matrix metalloproteinase-9 expression is mediated by integrins alpha5beta1 and alphavbeta5.
Milner R, Crocker SJ, Hung S, Wang X, Frausto RF, del Zoppo GJ. Milner R, et al. J Immunol. 2007 Jun 15;178(12):8158-67. doi: 10.4049/jimmunol.178.12.8158. J Immunol. 2007. PMID: 17548654 - Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures.
Rosenberg GA, Cunningham LA, Wallace J, Alexander S, Estrada EY, Grossetete M, Razhagi A, Miller K, Gearing A. Rosenberg GA, et al. Brain Res. 2001 Mar 2;893(1-2):104-12. doi: 10.1016/s0006-8993(00)03294-7. Brain Res. 2001. PMID: 11222998 - Effect of neutrophil depletion on gelatinase expression, edema formation and hemorrhagic transformation after focal ischemic stroke.
Harris AK, Ergul A, Kozak A, Machado LS, Johnson MH, Fagan SC. Harris AK, et al. BMC Neurosci. 2005 Aug 3;6:49. doi: 10.1186/1471-2202-6-49. BMC Neurosci. 2005. PMID: 16078993 Free PMC article. - Microglial activation and matrix protease generation during focal cerebral ischemia.
del Zoppo GJ, Milner R, Mabuchi T, Hung S, Wang X, Berg GI, Koziol JA. del Zoppo GJ, et al. Stroke. 2007 Feb;38(2 Suppl):646-51. doi: 10.1161/01.STR.0000254477.34231.cb. Stroke. 2007. PMID: 17261708 Review. - Profile of intravenous glyburide for the prevention of cerebral edema following large hemispheric infarction: evidence to date.
King ZA, Sheth KN, Kimberly WT, Simard JM. King ZA, et al. Drug Des Devel Ther. 2018 Aug 15;12:2539-2552. doi: 10.2147/DDDT.S150043. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30147301 Free PMC article. Review.
Cited by
- Genetic Deletion or Pharmacological Inhibition of Cyclooxygenase-2 Reduces Blood-Brain Barrier Damage in Experimental Ischemic Stroke.
Yang C, Yang Y, DeMars KM, Rosenberg GA, Candelario-Jalil E. Yang C, et al. Front Neurol. 2020 Aug 20;11:887. doi: 10.3389/fneur.2020.00887. eCollection 2020. Front Neurol. 2020. PMID: 32973660 Free PMC article. - Recommendations for preclinical research in hemorrhagic transformation.
Fagan SC, Lapchak PA, Liebeskind DS, Ishrat T, Ergul A. Fagan SC, et al. Transl Stroke Res. 2013 Jun;4(3):322-7. doi: 10.1007/s12975-012-0222-5. Transl Stroke Res. 2013. PMID: 23730351 Free PMC article. Review. - Stem cell recruitment of newly formed host cells via a successful seduction? Filling the gap between neurogenic niche and injured brain site.
Tajiri N, Kaneko Y, Shinozuka K, Ishikawa H, Yankee E, McGrogan M, Case C, Borlongan CV. Tajiri N, et al. PLoS One. 2013 Sep 4;8(9):e74857. doi: 10.1371/journal.pone.0074857. eCollection 2013. PLoS One. 2013. PMID: 24023965 Free PMC article. - Osteopontin expression in acute immune response mediates hippocampal synaptogenesis and adaptive outcome following cortical brain injury.
Chan JL, Reeves TM, Phillips LL. Chan JL, et al. Exp Neurol. 2014 Nov;261:757-71. doi: 10.1016/j.expneurol.2014.08.015. Epub 2014 Aug 21. Exp Neurol. 2014. PMID: 25151457 Free PMC article.
References
- Abilleira S, Montaner J, Molina CA, Monasterio J, Castillo J, Alvarez-Sabin J. Matrix metalloproteinase-9 concentration after spontaneous intracerebral hemorrhage. J Neurosurg. 2003;99:65–70. - PubMed
- Abumiya T, Lucero J, Heo JH, Tagaya M, Koziol JA, Copeland BR, del Zoppo GJ. Activated microvessels express vascular endothelial growth factor and integrin alpha(v)beta3 during focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:1038–1050. - PubMed
- Aoki T, Sumii T, Mori T, Wang X, Lo EH. Blood-brain barrier disruption and matrix metalloproteinase-9 expression during reperfusion injury: mechanical versus embolic focal ischemia in spontaneously hypertensive rats. Stroke. 2002;33:2711–2717. - PubMed
- Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS038710/NS/NINDS NIH HHS/United States
- R01 NS053716/NS/NINDS NIH HHS/United States
- NS 038710/NS/NINDS NIH HHS/United States
- R01 NS026945/NS/NINDS NIH HHS/United States
- R37 NS038710/NS/NINDS NIH HHS/United States
- NS 026945/NS/NINDS NIH HHS/United States
- NS 053716/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous