Lack of association of the S769N mutation in Plasmodium falciparum SERCA (PfATP6) with resistance to artemisinins - PubMed (original) (raw)
Lack of association of the S769N mutation in Plasmodium falciparum SERCA (PfATP6) with resistance to artemisinins
Long Cui et al. Antimicrob Agents Chemother. 2012 May.
Abstract
The recent emergence of artemisinin (ART) resistance in Plasmodium falciparum in western Cambodia, manifested as delayed parasite clearance, is a big threat to the long-term efficacy of this family of antimalarial drugs. Among the multiple candidate genes associated with ART resistance in P. falciparum, the sarcoplasmic/endoplasmic reticulum Ca(2+)-ATPase PfATP6 has been postulated as a specific target of ARTs. The PfATP6 gene harbors multiple single-nucleotide polymorphisms in field parasite populations, and S769N has been associated with decreased sensitivity to artemether in parasite populations from French Guiana. In this study, we used an allelic exchange strategy to engineer parasite lines carrying the S769N mutations in P. falciparum strain 3D7 and evaluated whether introduction of this mutation modulated parasite sensitivity to ART derivatives. Using three transgenic lines carrying the 769N mutation and two transgenic lines carrying the wild-type 769S as controls, we found that S769N did not affect PfATP6 gene expression. We compared the sensitivities of these parasite lines to three ART derivatives, artemether, artesunate, and dihydroartemisinin, in 18 biological experiments and detected no significant effect of the S769N mutation on parasite response to these ART derivatives. This study provides further evidence for the lack of association of PfATP6 with ART resistance.
Figures
Fig 1
Development of transgenic lines in 3D7 with the PfATP6 S769N mutation. (A) Schematic representation of single-crossover event at the Pfatp6 locus. (Top) The Pfatp6 locus on chromosome 1. Solid lines represent introns or intergenic regions, and filled boxes indicate the coding regions. (Middle) Plasmid pHD22y-pfatp6-769N, showing the Pfatp6 genomic fragment and the drug selection cassette hDHFR. (Bottom) Predicted single-crossover events at the Pfatp6 locus. The fragment within the bracket indicates the scenario when integration of concatemerized plasmid occurs. The C-terminal fragment of PfATP6 cloned in the transfection plasmid is shown as filled boxes. The open and filled lozenges indicate the locations of the wild-type and mutant amino acids at position 769, respectively. Restriction enzyme KpnI sites and the expected sizes of DNA fragments after KpnI digestion are illustrated. The positions and orientations of the primers on chromosome 1 and the plasmid are shown. Primer pairs P1 and P2 were used for integration-specific PCR, whereas primers P3 and P4 were used for determining the copy number of the integrated plasmid. The position of the probe used for genomic Southern blotting is also marked. (B) Integration-specific PCR products, based on used of primer P1 on chromosome 1 and primer P2 on the plasmid, showing 32 positive and 2 negative clones (lanes 28 and 32). The PCR products of 32 positive clones were sequenced, and asterisks indicate the three clones with the S769N mutation. (C) Copy numbers of the integrated plasmid or concatemers at the Pfatp6 locus as determined by real-time PCR using primer pairs P3 and P4. Shown here are five transgenic clones, with two containing the wild-type residue (739S-9 and 769S-17) and three with the 769N mutation (769N-2, 769N-7, and 769N-31). (D) Genomic Southern blot of DNA isolated from 3D7 and five clones with plasmid integration at the Pfatp6 locus. Genomic DNA was digested with KpnI and separated in a 1% agarose gel. The blot was hybridized with the probe marked in panel A, which revealed a ca. 10-kb fragment in 3D7 and 5.8-kb fragment in the recombinant Pfatp6 locus.
Fig 2
Pfatp6 expression in wild-type and S769N mutant clones. Pfatp6 expression levels are shown in the ring (12 h), trophozoite (30 h), and schizont (38 h) stages. The relative expression level of Pfatp6 was determined by real-time PCR analysis using primers P3 and P4. A housekeeping gene, seryl-tRNA synthetase (PF07_0073), was used as an internal control. There were no significant differences in mRNA levels among the parasite clones at each development time point (P > 0.05, ANOVA).
Fig 3
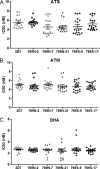
Scatter plot of IC50s of 3D7 and five transgenic clones carrying either 769N (2, 17, and 31) or 769S (9 and 17), assayed with ATM (top), ATS (middle), and DHA (bottom). Each value indicates the mean from three technical replicates. The means ± standard deviations were calculated from the means of 18 biological experiments. For statistical comparison, data were normalized using natural logarithm transformation. For each drug, there were no significant differences among the parasite lines after controlling for multiple tests (P > 0.05, unpaired t test).
Fig 4
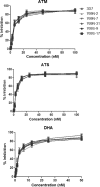
Dose-response curves of 3D7 and five parasite clones with either 769N (2, 17, and 31) or 769S (9 and 17) assayed against ATM (top), ATS (middle), and DHA (bottom). The results were obtained from 18 independent experiments, each with three technical replicates. Percent inhibition values are shown as means ± standard deviations.
Similar articles
- Artemether resistance in vitro is linked to mutations in PfATP6 that also interact with mutations in PfMDR1 in travellers returning with Plasmodium falciparum infections.
Pillai DR, Lau R, Khairnar K, Lepore R, Via A, Staines HM, Krishna S. Pillai DR, et al. Malar J. 2012 Apr 27;11:131. doi: 10.1186/1475-2875-11-131. Malar J. 2012. PMID: 22540925 Free PMC article. - Expression in yeast links field polymorphisms in PfATP6 to in vitro artemisinin resistance and identifies new inhibitor classes.
Pulcini S, Staines HM, Pittman JK, Slavic K, Doerig C, Halbert J, Tewari R, Shah F, Avery MA, Haynes RK, Krishna S. Pulcini S, et al. J Infect Dis. 2013 Aug 1;208(3):468-78. doi: 10.1093/infdis/jit171. Epub 2013 Apr 18. J Infect Dis. 2013. PMID: 23599312 - Genetic diversity and lack of artemisinin selection signature on the Plasmodium falciparum ATP6 in the Greater Mekong Subregion.
Miao M, Wang Z, Yang Z, Yuan L, Parker DM, Putaporntip C, Jongwutiwes S, Xangsayarath P, Pongvongsa T, Moji H, Dinh Tuong T, Abe T, Nakazawa S, Kyaw MP, Yan G, Sirichaisinthop J, Sattabongkot J, Mu J, Su XZ, Kaneko O, Cui L. Miao M, et al. PLoS One. 2013;8(3):e59192. doi: 10.1371/journal.pone.0059192. Epub 2013 Mar 26. PLoS One. 2013. PMID: 23555629 Free PMC article. - Considerations on the mechanism of action of artemisinin antimalarials: part 1--the 'carbon radical' and 'heme' hypotheses.
Haynes RK, Cheu KW, N'Da D, Coghi P, Monti D. Haynes RK, et al. Infect Disord Drug Targets. 2013 Aug;13(4):217-77. doi: 10.2174/1871526513666131129155708. Infect Disord Drug Targets. 2013. PMID: 24304352 Review. - Importance of kelch 13 C580Y mutation in the studies of artemisinin resistance in Plasmodium falciparum in Greater Mekong Subregion.
Zaw MT, Lin Z, Emran NA. Zaw MT, et al. J Microbiol Immunol Infect. 2020 Oct;53(5):676-681. doi: 10.1016/j.jmii.2019.07.006. Epub 2019 Sep 10. J Microbiol Immunol Infect. 2020. PMID: 31563454 Review.
Cited by
- Artemisinin-based combination therapy (ACT) and drug resistance molecular markers: A systematic review of clinical studies from two malaria endemic regions - India and sub-Saharan Africa.
Arya A, Kojom Foko LP, Chaudhry S, Sharma A, Singh V. Arya A, et al. Int J Parasitol Drugs Drug Resist. 2021 Apr;15:43-56. doi: 10.1016/j.ijpddr.2020.11.006. Epub 2020 Dec 13. Int J Parasitol Drugs Drug Resist. 2021. PMID: 33556786 Free PMC article. Review. - Molecular assessment of atpase6 mutations associated with artemisinin resistance among unexposed and exposed Plasmodium falciparum clinical isolates to artemisinin-based combination therapy.
Zakeri S, Hemati S, Pirahmadi S, Afsharpad M, Raeisi A, Djadid ND. Zakeri S, et al. Malar J. 2012 Nov 9;11:373. doi: 10.1186/1475-2875-11-373. Malar J. 2012. PMID: 23140394 Free PMC article. - Occurrence of pfatpase6 Single Nucleotide Polymorphisms Associated with Artemisinin Resistance among Field Isolates of Plasmodium falciparum in North-Eastern Tanzania.
Chilongola J, Ndaro A, Tarimo H, Shedrack T, Barthazary S, Kaaya R, Masokoto A, Kajeguka D, Kavishe RA, Lusingu J. Chilongola J, et al. Malar Res Treat. 2015;2015:279028. doi: 10.1155/2015/279028. Epub 2015 Jan 5. Malar Res Treat. 2015. PMID: 25685593 Free PMC article. - Monitoring artemisinin resistance in Plasmodium falciparum: comparison of parasite clearance time by microscopy and real-time PCR and evaluation of mutations in Pfatpase6 gene in Odisha state of India.
Gupta R, Mishra N, Kumar A, Rana R, Srivastava B, Tyagi PK, Anvikar AR, Valecha N. Gupta R, et al. Parasitol Res. 2015 Sep;114(9):3487-96. doi: 10.1007/s00436-015-4577-x. Epub 2015 Jun 27. Parasitol Res. 2015. PMID: 26113507 - The interplay between drug resistance and fitness in malaria parasites.
Rosenthal PJ. Rosenthal PJ. Mol Microbiol. 2013 Sep;89(6):1025-38. doi: 10.1111/mmi.12349. Epub 2013 Aug 16. Mol Microbiol. 2013. PMID: 23899091 Free PMC article. Review.
References
- Arnou B, et al. 2011. The Plasmodium falciparum Ca(2+)-ATPase PfATP6: insensitive to artemisinin, but a potential drug target. Biochem. Soc. Trans. 39:823–831 - PubMed
- Bhisutthibhan J, et al. 1998. The Plasmodium falciparum translationally controlled tumor protein homolog and its reaction with the antimalarial drug artemisinin. J. Biol. Chem. 273:16192–16198 - PubMed
- Bosman A, Mendis KN. 2007. A major transition in malaria treatment: the adoption and deployment of artemisinin-based combination therapies. Am. J. Trop. Med. Hyg. 77:193–197 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AI085518/AI/NIAID NIH HHS/United States
- U19AI089672/AI/NIAID NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- 1R21AI085518/AI/NIAID NIH HHS/United States
- U19 AI089672/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous