NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia - PubMed (original) (raw)
Review
NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia
Guillermo Gonzalez-Burgos et al. Schizophr Bull. 2012 Sep.
Abstract
Gamma oscillations appear to be dependent on inhibitory neurotransmission from parvalbumin (PV)-containing gamma-amino butyric acid neurons. Thus, the abnormalities in PV neurons found in schizophrenia may underlie the deficits of gamma-band synchrony in the illness. Because gamma-band synchrony is thought to be crucial for cognition, cognitive deficits in schizophrenia may reflect PV neuron dysfunction in cortical neural circuits. Interestingly, it has been suggested that PV alterations in schizophrenia are the consequence of a hypofunction of signaling through N-methyl-D-aspartate (NMDA) receptors (NMDARs). Here, we review recent findings that address the question of how NMDAR hypofunction might produce deficits of PV neuron-mediated inhibition in schizophrenia. We conclude that while dysregulation of NMDARs may play an important role in the pathophysiology of schizophrenia, additional research is required to determine the particular cell type(s) that mediate dysfunctional NMDAR signaling in the illness.
Figures
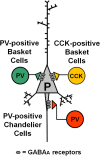
Fig. 1.
Major subtypes of perisomatic-targeting gamma-amino butyric acid (GABA) neurons in cortical circuits. Both parvalbumin (PV)-positive and cholecystokinin (CCK)-positive basket cells (green and yellow, respectively) target the soma and proximal dendrites of the pyramidal cell membrane. PV-positive chandelier cells (red) target the axon initial segment. These 3 GABA neuron subtypes signal via GABAA receptors, although different GABAA receptor subtypes may mediate the response to GABA at each type of synapse (not shown in the scheme). As described in the main text, current data suggest that gamma oscillation production depends mostly on pyramidal neuron inhibition by PV-positive basket cells.
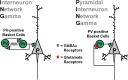
Fig. 2.
Two different circuit-based models for gamma oscillation production. In the Interneuron Network Gamma (ING) model (left panel), the oscillation depends on reciprocal inhibitory interactions between parvalbumin (PV)-positive basket cells. Some form of continuous (tonic) excitatory current is the main source of interneuron activation in ING. Important for the ING model are the electrical gap-junction connections (zig-zag wires) between PV-positive basket cells. In ING, pyramidal cells are synchronized rhythmically by the interneuron network but are not directly involved in rhythm production. In the Pyramidal Interneuron Network Gamma (PING) model (right panel), oscillations depend on the interplay between pyramidal cells and gamma-amino butyric acid neurons via recurrent synaptic connections. In PING models, interneurons are driven by the phasic (synaptic) excitatory glutamate–mediated currents arriving from the pyramidal cells, which are synchronized rhythmically by feedback inhibition. Monosynaptic excitation from pyramidal cells is the main source of interneuron activation in PING. Therefore, in PING, pyramidal cells are directly involved in the gamma rhythm mechanisms. As reviewed in the main text, some current data favor the PING over the ING model.
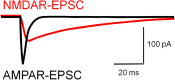
Fig. 3.
Properties of _N_-methyl-
d
-aspartate receptor (NMDAR)- and AMPAR-mediated excitatory synaptic currents (EPSCs). Various properties distinguishing NMDAR-EPSCs and AMPAR-EPSCs are described in the main text. Especially, important for models of gamma oscillations is the much shorter duration of the AMPAR-EPSC relative to the NMDAR-EPSC. Whereas short-lasting AMPAR-EPSCs are sufficient to support robust gamma oscillations, long-lasting NMDAR-EPSCs produce asynchronous and delayed postsynaptic interneuron firing that is not locked to the incoming inputs from pyramidal cells and in a PING model of gamma oscillations reduces gamma oscillation power. Such an inverse relation between gamma power and NMDAR contribution suggests that the reduction of NMDAR contribution at synapses onto parvalbumin (PV)-positive basket cells that occurs during normal postnatal development, helps optimize gamma oscillation production during cortical circuit development. Thus, during gamma oscillations in adult cortex, PV-positive basket cells appear to be mostly driven by fast AMPAR-mediated excitation. See main text for additional details.
Similar articles
- GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia.
Gonzalez-Burgos G, Fish KN, Lewis DA. Gonzalez-Burgos G, et al. Neural Plast. 2011;2011:723184. doi: 10.1155/2011/723184. Epub 2011 Sep 5. Neural Plast. 2011. PMID: 21904685 Free PMC article. Review. - Abnormal Gamma Oscillations in N-Methyl-D-Aspartate Receptor Hypofunction Models of Schizophrenia.
Jadi MP, Behrens MM, Sejnowski TJ. Jadi MP, et al. Biol Psychiatry. 2016 May 1;79(9):716-726. doi: 10.1016/j.biopsych.2015.07.005. Epub 2015 Jul 17. Biol Psychiatry. 2016. PMID: 26281716 Free PMC article. Review. - Gamma oscillation deficits and the onset and early progression of schizophrenia.
Woo TU, Spencer K, McCarley RW. Woo TU, et al. Harv Rev Psychiatry. 2010 May-Jun;18(3):173-89. doi: 10.3109/10673221003747609. Harv Rev Psychiatry. 2010. PMID: 20415633 Free PMC article. - Cortical basket cell dysfunction in schizophrenia.
Curley AA, Lewis DA. Curley AA, et al. J Physiol. 2012 Feb 15;590(4):715-24. doi: 10.1113/jphysiol.2011.224659. Epub 2012 Jan 4. J Physiol. 2012. PMID: 22219337 Free PMC article. Review. - Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia.
Gonzalez-Burgos G, Cho RY, Lewis DA. Gonzalez-Burgos G, et al. Biol Psychiatry. 2015 Jun 15;77(12):1031-40. doi: 10.1016/j.biopsych.2015.03.010. Epub 2015 Mar 17. Biol Psychiatry. 2015. PMID: 25863358 Free PMC article. Review.
Cited by
- Functional connectivity signatures of NMDAR dysfunction in schizophrenia-integrating findings from imaging genetics and pharmaco-fMRI.
Gaebler AJ, Fakour N, Stöhr F, Zweerings J, Taebi A, Suslova M, Dukart J, Hipp JF, Adhikari BM, Kochunov P, Muthukumaraswamy SD, Forsyth A, Eggermann T, Kraft F, Kurth I, Paulzen M, Gründer G, Schneider F, Mathiak K. Gaebler AJ, et al. Transl Psychiatry. 2023 Feb 16;13(1):59. doi: 10.1038/s41398-023-02344-2. Transl Psychiatry. 2023. PMID: 36797233 Free PMC article. Clinical Trial. - Parvalbumin expression and gamma oscillation occurrence increase over time in a neurodevelopmental model of NMDA receptor dysfunction.
van Lier B, Hierlemann A, Knoflach F. van Lier B, et al. PeerJ. 2018 Sep 19;6:e5543. doi: 10.7717/peerj.5543. eCollection 2018. PeerJ. 2018. PMID: 30258707 Free PMC article. - Detection of Multiway Gamma Coordination Reveals How Frequency Mixing Shapes Neural Dynamics.
Haufler D, Paré D. Haufler D, et al. Neuron. 2019 Feb 20;101(4):603-614.e6. doi: 10.1016/j.neuron.2018.12.028. Epub 2019 Jan 21. Neuron. 2019. PMID: 30679018 Free PMC article. - Directly and Indirectly Targeting the Glycine Modulatory Site to Modulate NMDA Receptor Function to Address Unmet Medical Needs of Patients With Schizophrenia.
Pei JC, Luo DZ, Gau SS, Chang CY, Lai WS. Pei JC, et al. Front Psychiatry. 2021 Oct 1;12:742058. doi: 10.3389/fpsyt.2021.742058. eCollection 2021. Front Psychiatry. 2021. PMID: 34658976 Free PMC article. Review. - Effects of Ketamine on Basal Gamma Band Oscillation and Sensory Gating in Prefrontal Cortex of Awake Rats.
Qi R, Li J, Wu X, Geng X, Chen N, Yu H. Qi R, et al. Neurosci Bull. 2018 Jun;34(3):457-464. doi: 10.1007/s12264-018-0208-8. Epub 2018 Jan 29. Neurosci Bull. 2018. PMID: 29380249 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical