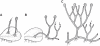Major transitions in the evolution of early land plants: a bryological perspective - PubMed (original) (raw)
Review
. 2012 Apr;109(5):851-71.
doi: 10.1093/aob/mcs017. Epub 2012 Feb 22.
Affiliations
- PMID: 22356739
- PMCID: PMC3310499
- DOI: 10.1093/aob/mcs017
Review
Major transitions in the evolution of early land plants: a bryological perspective
Roberto Ligrone et al. Ann Bot. 2012 Apr.
Abstract
Background Molecular phylogeny has resolved the liverworts as the earliest-divergent clade of land plants and mosses as the sister group to hornworts plus tracheophytes, with alternative topologies resolving the hornworts as sister to mosses plus tracheophytes less well supported. The tracheophytes plus fossil plants putatively lacking lignified vascular tissue form the polysporangiophyte clade. Scope This paper reviews phylogenetic, developmental, anatomical, genetic and paleontological data with the aim of reconstructing the succession of events that shaped major land plant lineages. Conclusions Fundamental land plant characters primarily evolved in the bryophyte grade, and hence the key to a better understanding of the early evolution of land plants is in bryophytes. The last common ancestor of land plants was probably a leafless axial gametophyte bearing simple unisporangiate sporophytes. Water-conducting tissue, if present, was restricted to the gametophyte and presumably consisted of perforate cells similar to those in the early-divergent bryophytes Haplomitrium and Takakia. Stomata were a sporophyte innovation with the possible ancestral functions of producing a transpiration-driven flow of water and solutes from the parental gametophyte and facilitating spore separation before release. Stomata in mosses, hornworts and polysporangiophytes are viewed as homologous, and hence these three lineages are collectively referred to as the 'stomatophytes'. An indeterminate sporophyte body (the sporophyte shoot) developing from an apical meristem was the key innovation in polysporangiophytes. Poikilohydry is the ancestral condition in land plants; homoiohydry evolved in the sporophyte of polysporangiophytes. Fungal symbiotic associations ancestral to modern arbuscular mycorrhizas evolved in the gametophytic generation before the separation of major present-living lineages. Hydroids are imperforate water-conducting cells specific to advanced mosses. Xylem vascular cells in polysporangiophytes arose either from perforate cells or de novo. Food-conducting cells were a very early innovation in land plant evolution. The inferences presented here await testing by molecular genetics.
Figures
Fig. 1.
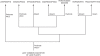
Cladogram of land plants (based on Kenrick and Crane, 1997_a_; Heinrichs et al., 2005; Forrest et al., 2006; Qiu et al., 2006; Cox et al., 2010; Gerrienne and Gonez, 2011).
Fig. 2.
Extant members of bryophyte lineages. (A, B) Monoclea forsteri (complex thalloid liverworts), male gametophytes (A) and mature sporophytes (B). (C) Schistochila alata (leafy liverworts), gametophytes. (D) Polytrichastrum formosum (mosses), mature gametophytes and sporophytes. (E) Phaeoceros carolinianus (hornworts), gametophytes and sporophytes.
Fig. 3.
Diagrammatic representation of sporophyte development in liverworts, mosses and hornworts. The bicellular stage in hornworts is not in colour as the first two cells are not yet differentially determined. Different scale sizes are used for the zygote and early-embryo stages relative to later stages. Although most likely homologous with the foot in liverworts and mosses, the hornwort foot is illustrated in a different colour because differences in early embryo development do not distinguish epi- and hypobasally derived parts.
Fig. 4.
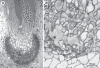
Details of the sporophyte in the hornwort Phaeomegaceros coriaceus. (A) Sporophyte base showing the foot (f) embedded in gametophyte tissue (g) and the persistent basal meristem (m). (B) Higher magnification of the placental region showing sporophytic haustorial cells (arrows) and gametophytic transfer cells (g).
Fig. 5.
Dichotomously branched polysporangiate sporophyte of Psilotum nudum. In spite of its superficial resemblance to fossil rhyniopsids, Psilotum is firmly nested within the monilophytes along with the ferns and horsetails (Qiu et al., 2006, 2007).
Fig. 6.
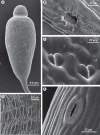
Diversity of stomatal morphology in bryophytes. (A) Young sporangium of the moss Bryum capillare with numerous stomata scattered in the neck region. (B) Detail of sporangial wall in the moss Orthotrichum anomalum, showing a sunken stoma overarched by projections from adjacent epidermal cells. (C) Non-functional stomata in the moss Sphagnum fimbriatum. (D) Densely arranged stomata raised above the epidermis in the sporangium of the moss Polytrichastrum formosum. (E) A stoma in the sporangial wall of Anthoceros punctatus; the guard cells are covered with hydrophobic material that possibly prevents water entry into the substomatal cavity.
Fig. 7.
Transmission electron micrographs of sporophyte seta in the moss Oedipodium griffithianum. (A) Transverse section showing thick-walled peripheral cells. The arrows point to a cuticle-like coat on the external surface. (B) At higher magnification the cuticle-like coat shows a bilayered structure (arrows).
Fig. 8.
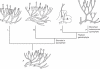
Reconstruction of the divergence of the liverwort (L), moss (M), hornwort (H) and polysporangiophyte (P) lineages from a common ancestor (A) represented as a dichotomously branched, leafless gametophyte with rhizoid-producing horizontal axes and erect photosynthetic axes bearing simple terminal sporophytes. Mosses, hornworts and polysporangiophytes (M, H and P) form the stomatophyte clade. Splitting of mature sporangia is shown in (A) and (H). The gametophyte generation has been omitted for the polysporangiophyte lineage (P). Black dots on sporophytes in M, H and P are stomata. Cladogram based on Qiu et al. (2006).
Fig. 9.
Hypothetical stages in the evolution of polysporangiophytes. (A) Unbranched sporophyte permanently attached to a thalloid gametophyte. (B) Branched sporophyte retaining attachment to the gametophyte but also producing rhizoid-bearing axes. (C) Profusely branched sporophyte attaining autonomy early in development. The gametophyte generation is omitted in C. Black dots on sporophyte axes and sporangia are stomata. Arrows point to apical meristem in sporophyte vegetative axes.
Fig. 10.
Distribution of water-conducting cells (WCC) in embryophytes. Cladogram based on Qiu et al. (2007) and Cox et al. (2010).
Fig. 11.
Transverse sections of the leafy shoot in the moss Polytrichastrum formosum showing reversible collapse and expansion of hydroids: (A) hydrated state, (B) dehydrated state.
Similar articles
- The origin of the sporophyte shoot in land plants: a bryological perspective.
Ligrone R, Duckett JG, Renzaglia KS. Ligrone R, et al. Ann Bot. 2012 Oct;110(5):935-41. doi: 10.1093/aob/mcs176. Epub 2012 Aug 7. Ann Bot. 2012. PMID: 22875816 Free PMC article. - Vegetative and reproductive innovations of early land plants: implications for a unified phylogeny.
Renzaglia KS, Duff RJT, Nickrent DL, Garbary DJ. Renzaglia KS, et al. Philos Trans R Soc Lond B Biol Sci. 2000 Jun 29;355(1398):769-93. doi: 10.1098/rstb.2000.0615. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 10905609 Free PMC article. Review. - The hornworts: morphology, evolution and development.
Frangedakis E, Shimamura M, Villarreal JC, Li FW, Tomaselli M, Waller M, Sakakibara K, Renzaglia KS, Szövényi P. Frangedakis E, et al. New Phytol. 2021 Jan;229(2):735-754. doi: 10.1111/nph.16874. Epub 2020 Sep 15. New Phytol. 2021. PMID: 32790880 Free PMC article. Review. - How was apical growth regulated in the ancestral land plant? Insights from the development of non-seed plants.
Fouracre JP, Harrison CJ. Fouracre JP, et al. Plant Physiol. 2022 Aug 29;190(1):100-112. doi: 10.1093/plphys/kiac313. Plant Physiol. 2022. PMID: 35771646 Free PMC article. Review. - Bryophyte diversity and evolution: windows into the early evolution of land plants.
Shaw AJ, Szövényi P, Shaw B. Shaw AJ, et al. Am J Bot. 2011 Mar;98(3):352-69. doi: 10.3732/ajb.1000316. Epub 2011 Feb 25. Am J Bot. 2011. PMID: 21613131 Review.
Cited by
- Genome-wide transcriptomic analysis of the sporophyte of the moss Physcomitrella patens.
O'Donoghue MT, Chater C, Wallace S, Gray JE, Beerling DJ, Fleming AJ. O'Donoghue MT, et al. J Exp Bot. 2013 Sep;64(12):3567-81. doi: 10.1093/jxb/ert190. Epub 2013 Jul 25. J Exp Bot. 2013. PMID: 23888066 Free PMC article. - Conserved Gene Expression Programs in Developing Roots from Diverse Plants.
Huang L, Schiefelbein J. Huang L, et al. Plant Cell. 2015 Aug;27(8):2119-32. doi: 10.1105/tpc.15.00328. Epub 2015 Aug 11. Plant Cell. 2015. PMID: 26265761 Free PMC article. - Archetypal Roles of an Abscisic Acid Receptor in Drought and Sugar Responses in Liverworts.
Jahan A, Komatsu K, Wakida-Sekiya M, Hiraide M, Tanaka K, Ohtake R, Umezawa T, Toriyama T, Shinozawa A, Yotsui I, Sakata Y, Takezawa D. Jahan A, et al. Plant Physiol. 2019 Jan;179(1):317-328. doi: 10.1104/pp.18.00761. Epub 2018 Nov 15. Plant Physiol. 2019. PMID: 30442644 Free PMC article. - Genetic analysis of DEFECTIVE KERNEL1 loop function in three-dimensional body patterning in Physcomitrella patens.
Demko V, Perroud PF, Johansen W, Delwiche CF, Cooper ED, Remme P, Ako AE, Kugler KG, Mayer KF, Quatrano R, Olsen OA. Demko V, et al. Plant Physiol. 2014 Oct;166(2):903-19. doi: 10.1104/pp.114.243758. Epub 2014 Sep 2. Plant Physiol. 2014. PMID: 25185121 Free PMC article. - Mechanisms of abscisic acid-mediated control of stomatal aperture.
Munemasa S, Hauser F, Park J, Waadt R, Brandt B, Schroeder JI. Munemasa S, et al. Curr Opin Plant Biol. 2015 Dec;28:154-62. doi: 10.1016/j.pbi.2015.10.010. Epub 2015 Nov 19. Curr Opin Plant Biol. 2015. PMID: 26599955 Free PMC article. Review.
References
- Albert VA. Shoot apical meristems and floral patterning: an evolutionary perspective. Trends in Plant Science. 1999;4:84–86.
- Alpert P. The limits and frontiers of desiccation-tolerant life. Integrative and Comparative Biology. 2005;45:685–695. - PubMed
- Asakawa Y. Chemical constituents of the Bryophytes. In: Herz W, Kirby GW, Moore RE, Steglich W, editors. Progress in the chemistry of organic natural products. Vol. 65. Vienna: Springer; 1995. pp. 1–562. - PubMed
- Bargel H, Koch K, Cerman Z, Neinhuis C. Structure-function relationships of the plant cuticle and cuticular waxes – a smart material? Functional Plant Biology. 2006;33:893–910. - PubMed
- Barnett JR. Plasmodesmata and pit development in secondary xylem elements. Planta. 1982;155:251–260. - PubMed