The role of gut microbiota in immune homeostasis and autoimmunity - PubMed (original) (raw)
Review
. 2012 Jan-Feb;3(1):4-14.
doi: 10.4161/gmic.19320. Epub 2012 Jan 1.
Affiliations
- PMID: 22356853
- PMCID: PMC3337124
- DOI: 10.4161/gmic.19320
Review
The role of gut microbiota in immune homeostasis and autoimmunity
Hsin-Jung Wu et al. Gut Microbes. 2012 Jan-Feb.
Abstract
Keeping a delicate balance in the immune system by eliminating invading pathogens, while still maintaining self-tolerance to avoid autoimmunity, is critical for the body's health. The gut microbiota that resides in the gastrointestinal tract provides essential health benefits to its host, particularly by regulating immune homeostasis. Moreover, it has recently become obvious that alterations of these gut microbial communities can cause immune dysregulation, leading to autoimmune disorders. Here we review the advances in our understanding of how the gut microbiota regulates innate and adaptive immune homeostasis, which in turn can affect the development of not only intestinal but also systemic autoimmune diseases. Exploring the interaction of gut microbes and the host immune system will not only allow us to understand the pathogenesis of autoimmune diseases but will also provide us new foundations for the design of novel immuno- or microbe-based therapies.
Figures
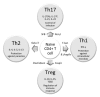
Figure 1. Commensal bacteria induce CD4+T cell differentiation. Naïve CD4+T cells can differentiate into four major cell types: Th1, Th2, Tregs and Th17. The differentiation of each lineage requires the induction of a transcription factor that is unique to each lineage. Once differentiated, each lineage secretes a special (set of) cytokine, as shown in the figure. Th1 cells play an important role in eliminating intracellular pathogens while Th2 function to control parasitic infection. The primary role of Th17 is to control infection and Tregs is to regulate immune response. The type of bacteria species that has been shown to induce a particular T cell differentiation pathway is indicated in the figure.
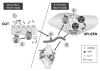
Figure 2. An autoimmune arthritis model that demonstrates the link between gut microbiota and an extraintestinal disease. The K/BxN arthritis model was used to demonstrate how the gut microbiota can influence a non-gut-associated disease. K/BxN mice express a transgene-encoded T-cell receptor that reacts to a self-peptide. Colonization of SFB on the gut induces the differentiation of Th17 cells (step 1 and 2), which subsequently exit the gut and migrate into the peripheral lymphoid tissue. The gut-origin of Th17 cells can be identified by their expression of the α4β7 receptor, imprinted on these T cells by intestinal-mucosa-associated DCs (step 3). IL-17, in turn, acts directly on B cells to provide help in the differentiation of germinal center B cells and the production of autoantibody in spleen (step 4). The autoantibody then circulates into its target organ joints, which ultimately leads to the development of disease.
Similar articles
- Microbiota-stimulated immune mechanisms to maintain gut homeostasis.
Chung H, Kasper DL. Chung H, et al. Curr Opin Immunol. 2010 Aug;22(4):455-60. doi: 10.1016/j.coi.2010.06.008. Epub 2010 Jul 23. Curr Opin Immunol. 2010. PMID: 20656465 Review. - Host-microbiota interplay in mediating immune disorders.
Felix KM, Tahsin S, Wu HJ. Felix KM, et al. Ann N Y Acad Sci. 2018 Apr;1417(1):57-70. doi: 10.1111/nyas.13508. Epub 2017 Oct 6. Ann N Y Acad Sci. 2018. PMID: 28984367 Free PMC article. Review. - Microbiota and autoimmunity.
Chervonsky AV. Chervonsky AV. Cold Spring Harb Perspect Biol. 2013 Mar 1;5(3):a007294. doi: 10.1101/cshperspect.a007294. Cold Spring Harb Perspect Biol. 2013. PMID: 23457255 Free PMC article. Review. - The interplay between the gut microbiota and the immune system.
Geuking MB, Köller Y, Rupp S, McCoy KD. Geuking MB, et al. Gut Microbes. 2014 May-Jun;5(3):411-8. doi: 10.4161/gmic.29330. Epub 2014 Jun 12. Gut Microbes. 2014. PMID: 24922519 Free PMC article. Review. - Microbiota-immune system interaction: an uneasy alliance.
Salzman NH. Salzman NH. Curr Opin Microbiol. 2011 Feb;14(1):99-105. doi: 10.1016/j.mib.2010.09.018. Epub 2010 Oct 21. Curr Opin Microbiol. 2011. PMID: 20971034 Free PMC article. Review.
Cited by
- The Role of Gut Microbiota in the Efficacy and Side Effect Profile of Biologic Therapies for Autoimmune Diseases.
Qusty N, Sarhan A, Taha M, Alshanqiti A, Almuteb AM, Alfaraidi AT, Alkhairi HA, Alzahrani MM, Alamry AHA, Alomry TQB, Bannan OA, Almaashi MS. Qusty N, et al. Cureus. 2024 Oct 8;16(10):e71111. doi: 10.7759/cureus.71111. eCollection 2024 Oct. Cureus. 2024. PMID: 39525264 Free PMC article. Review. - Effect of Probiotics on Gastrointestinal Health Through the Aryl Hydrocarbon Receptor Pathway: A Systematic Review.
De la Rosa González A, Guerra-Ojeda S, Camacho-Villa MA, Valls A, Alegre E, Quintero-Bernal R, Martorell P, Chenoll E, Serna-García M, Mauricio MD, Serna E. De la Rosa González A, et al. Foods. 2024 Oct 30;13(21):3479. doi: 10.3390/foods13213479. Foods. 2024. PMID: 39517263 Free PMC article. Review. - Experiences of discrimination are associated with microbiome and transcriptome alterations in the gut.
Dong TS, Shera S, Peters K, Gee GC, Beltrán-Sánchez H, Wang MC, Kilpatrick LA, Zhang X, Labus JS, Vaughan A, Church A. Dong TS, et al. Front Microbiol. 2024 Oct 24;15:1457028. doi: 10.3389/fmicb.2024.1457028. eCollection 2024. Front Microbiol. 2024. PMID: 39512934 Free PMC article. - Gut microbiota and autoimmune diseases: Insights from Mendelian randomization.
Mu F, Rusip G, Florenly F. Mu F, et al. FASEB Bioadv. 2024 Sep 21;6(11):467-476. doi: 10.1096/fba.2024-00037. eCollection 2024 Nov. FASEB Bioadv. 2024. PMID: 39512840 Free PMC article. Review. - Western diet promotes endometriotic lesion growth in mice and induces depletion of Akkermansia muciniphila in intestinal microbiota.
Parpex G, Chassaing B, Bourdon M, Santulli P, Doridot L, Thomas M, Batteux F, Chouzenoux S, Chapron C, Nicco C, Marcellin L. Parpex G, et al. BMC Med. 2024 Nov 6;22(1):513. doi: 10.1186/s12916-024-03738-9. BMC Med. 2024. PMID: 39501247 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical