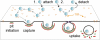Limited transferrin receptor clustering allows rapid diffusion of canine parvovirus into clathrin endocytic structures - PubMed (original) (raw)
Limited transferrin receptor clustering allows rapid diffusion of canine parvovirus into clathrin endocytic structures
David K Cureton et al. J Virol. 2012 May.
Abstract
Viral pathogens usurp cell surface receptors to access clathrin endocytic structures, yet the mechanisms of virus incorporation into these structures remain incompletely understood. Here we used fluorescence microscopy to directly visualize the association of single canine parvovirus (CPV) capsids with cellular transferrin receptors (TfR) on the surfaces of live feline cells and to monitor how these CPV-TfR complexes access endocytic structures. We found that most capsids associated with fewer than five TfRs and that ∼25% of TfR-bound capsids laterally diffused into assembling clathrin-coated pits less than 30 s after attachment. Capsids that did not encounter a coated pit dissociated from the cell surface with a half-life of ∼30 s. Together, our results show how CPV exploits the natural mechanism of TfR endocytosis to engage the clathrin endocytic pathway and reveal that the low affinity of capsids for feline TfRs limits the residence time of capsids on the cell surface and thus the efficiency of virus internalization.
Figures
Fig 1
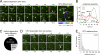
CPV association with clathrin endocytic structures in feline cells. (A) Clathrin-coated vesicle formation in CRFK cells. Left, image of a CRFK σ2-eGFP cell extracted from frame 1 of a 10-min time-lapse acquisition. Images were captured at 3-s intervals using a spinning disk confocal microscope. Right, kymograph view of clathrin-coated vesicle formation by the cell shown at left. (B) Fluorescence intensity of Alexa 647 CPV capsids. Confocal images of labeled capsids on glass were acquired, and the fluorescence intensity of each capsid spot was quantified. Inset, enlarged view of capsids (red) on glass. (C) Colocalization of CPV capsids with clathrin-coated pits (see Movie S1 in the supplemental material). CRFK σ2-eGFP cells were inoculated with Alexa 647 capsids and imaged as for panel A. A merge of the AP-2 (green) and capsid (red) channels is shown, along with an enlarged view of 2 capsids in coated pits from the boxed region at left. Arrowheads highlight capsids in (yellow) or outside (red) coated pits.
Fig 2
Real-time imaging of clathrin-dependent CPV internalization. (A) Example of clathrin-dependent CPV endocytosis (see Movie S2 in the supplemental material). Left panels, CRFK σ2-eGFP cells (green, AP-2) were inoculated with fluorescent capsids (red) and imaged as before. The image frame prior to capsid attachment was set to t = 0 s. The capsid attaches in frame +3 and diffuses laterally on the cell surface. The coated pit that is the future site of capsid uptake initiates in frame +33, while the capsid is still undergoing diffusion. The capsid collides with the assembling pit in frame +51, and the capsid and pit signals remain colocalized, signifying capsid incorporation into the pit. Frame +87 depicts the capsid-containing pit during the process of internalization (note the decrease in the capsid and AP-2 fluorescence). Right panel, diffusion path of the capsid shown at left. A color-coded line trace of the capsid diffusion path is overlaid onto the t = 51-s image. (B) Plot of the background-corrected AP-2 (green) and capsid (red) fluorescence intensities with respect to time for the event in panel A. For frames prior to pit initiation, the AP-2 fluorescence intensity was quantified at the eventual site of pit initiation. (C) Efficiency of clathrin-dependent CPV entry. Efficiency is expressed as a percentage of the total particles that bound to cells during time-lapse imaging of 4 cells. The percentage of particles that entered by clathrin-dependent endocytosis was 8% (9 entering/108 total), 30% (38/125), 24% (52/220), and 27% (48/175). (D) Examples of CPV dissociation from the cell surface (see Movie S3 in the supplemental material). Time-lapse images showing the attachment (downward-facing arrows) of two capsids (red; no. 1, no. 2) and subsequent capsid dissociation (upward-facing arrows). (E) Residence time of CPV capsids that dissociated from CRFK cells. The elapsed time between capsid attachment and disappearance was measured for capsids that remained bound for ≥6 s. Events are from the same 4 cells that were analyzed for panel C.
Fig 3
CPV capsids diffuse into newly formed clathrin-coated pits. (A) Schematics depicting how CPV capsids incorporate into coated pits. For each clathrin-dependent CPV entry event, the process of capsid-pit association was analyzed and recorded. The events were assigned to one of the four observed categories (numbered 1 to 4). (B) Chart showing the frequency of each capture mode. Data are from 18 cells. The data set(s) that comprises each slice is indicated according to the nomenclature in panel A. (C) The time interval between particle attachment and capture by a coated pit. The mean time to capture was 15 ± 14 s. (D and E) Timing of CPV capture expressed relative to the time of coated pit initiation (D) or as a percentage of the total pit lifetime (E). In panel E, 0% = pit initiation; 100% = loss of adaptor signal.
Fig 4
CPV does not alter the assembly kinetics or adaptor content of coated pits. Confocal images of CRFK σ2-eGFP cells were acquired at 3-s intervals. (A) Total lifetime of conventional coated pits (left) or pits that internalized a CPV capsid (right). Data for standard coated pits are from 4 individual cells in the presence of CPV, and data for CPV-containing pits are from 17 cells and include events from data sets no. 1 and 2 (Fig. 3A). The average lifetimes of standard coated pits (54 ± 19 s) and CPV-containing pits (53 ± 19 s) are not significantly different (two-tailed Student's t test, P = 0.33). (B) Lifetime versus maximum AP-2 fluorescence of standard (left) or CPV-containing (right) coated pits. Data for standard coated pits are from 4 cells. CPV data are from the same 4 cells and include events in data sets no. 1 and 2. Each open circle represents a single coated pit. The average peaks of fluorescence of AP-2 in standard coated pits (43 × 103 ± 1.8 × 103) and CPV-containing pits (45 × 103 ± 2.0 × 103) are not significantly different (two-tailed Student's t test, P = 0.44).
Fig 5
Receptor-bound CPV capsids diffuse slower than single TfRs. (A) CPV diffusion and uptake imaged by TIRFM. CRFK σ2-eGFP cells were inoculated with fluorescent CPV capsids, and images were captured at 1-s intervals. Left, frame 194 of a 5-min time-lapse acquisition showing capsids (red) present on the free cell surface (red arrowheads) or colocalizing with AP-2 (green) in coated pits (yellow or blue arrowheads). The inset shows a zoomed view of two capsids in coated pits from the boxed region below. Right, kymograph view of coated pit formation over time in the cell shown at left. The blue arrowhead highlights internalization of the same capsid marked with a blue arrowhead at left. A zoomed view of the event is provided below. (B) Example CPV internalization event observed by TIRFM (see Movie S4 in the supplemental material). Frames prior to virus capture are spaced at 1-s intervals, and frames after virus capture are spaced at 3-s intervals. Scale bar, 1 μm. (C) Example traces of capsid diffusion. Traces of four capsids are displayed in separate colors. The start of each trace is centered at (x = 0, y = 0). Small points indicate the particle position in sequential frames spaced at 1-s intervals. Large dots indicate the location of particle capture by a coated pit. The light-blue trace corresponds to the diffusion path of the capsid highlighted by a blue arrowhead in panels A and B. (D) Mean square displacement plots for the objects shown in panel C. The diffusion coefficient (D) for each capsid is provided. Plot colors refer to traces of the same color in panel C. (E) Diffusion coefficients of receptor-bound Tf (gray; see Movie S5 in the supplemental material) or CPV (red). Gray bars designate the mean for each population. Tf and CPV data are from 6 and 8 cells, respectively.
Fig 6
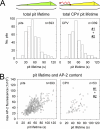
CPV capsids cross-link TfRs on the surfaces of live cells. (A) Schematics of fTfR tagged with eGFP. The eGFP molecule was appended to the C-terminal ectodomain of the fTfR such that each receptor dimer contains two eGFPs. (B) Images of CPV capsids colocalizing with fTfR-eGFP in endosomal compartments. TRVb cells stably expressing fTfR-eGFP (green) were inoculated with Atto 647 CPV capsids (red) and incubated at 37°C for 10 min. Live cells were imaged by TIRFM, and a representative image from a time-lapse acquisition is shown. Right-hand panels show an expanded view of the boxed region at left. The fTfR-eGFP channel is shown as a merge with the Atto 647 CPV channel (top) or alone (bottom). Arrowheads highlight the receptor signal in endosomes that contain CPV. Scale bar in right panels, 1 μm. (C) Comparison of CPV binding and uptake in CRFK or TRVb fTfR-eGFP cells. Cells were inoculated with Alexa 647 CPV capsids at t = 0 and 37°C, and the cell-associated capsid fluorescence was quantified by flow cytometry at the indicated times for the receptor-expressing cells. At all times, 2-5-fold more capsids associated with TRVb fTfR-eGFP cells than with CRFK cells. To compare the rates of capsid association with both cell types, we expressed the raw fluorescence values as a fraction of the values obtained at 20 min postinoculation (p.i.) in each cell type. Data points are the means ± SD for 3 experiments. Asterisks indicate time points when the difference in the mean values is statistically significant (two-tailed Student's t test, P < 0.05). (D) Schematic illustrating the position dependence of CPV-fTfR-eGFP complex detection using TIRFM. The intensity of the evanescent wave (gray) generated from the totally internally reflected laser source decreases exponentially as the distance from the coverslip increases. Thus, CPV capsids (red) and the associated fTfR-eGFP molecules (green) are maximally excited when directly contacting the glass coverslip, but those on the upper cell surface are excited to a lesser extent and therefore emit a lower fluorescent signal. (E) Example of receptor engagement by a CPV capsid (see Movie S7 in the supplemental material). TRVb fTfR-eGFP cells were inoculated with Atto 647 CPV capsids, and images were acquired at 2-s intervals using TIRFM. Left, images showing attachment of a CPV capsid (red) and the spot of fTfR-eGFP (green, white arrowheads) that colocalizes with the particle. In each panel, the CPV channel was shifted to the left by 6 pixels to reveal the underlying receptor signal. Right, plot of the CPV and TfR fluorescence intensity for the images at left. (F) Box plots showing the fluorescence of Atto 647 CPV capsids or single eGFP molecules on glass compared to their signals in CPV-fTfR-eGFP complexes on the cell surface. Glass data for eGFP molecules and CPV are from Fig. S2 in the supplemental material, panels C and D. Cell data are from 76 events observed on 19 cells. Upper and lower bounds of boxes correspond to the 75th and 25th percentiles, while error bar whiskers show the 90th and 10th percentiles of the data. Red and black horizontal lines indicate the mean and median values of the data sets. The raw fluorescence intensity of fTfR-eGFP associated with each capsid on the cell surface (middle bar, right panel) was multiplied by a correction factor of 3.5 (right panel), derived from the difference in the median fluorescence intensities of capsids on glass compared to those on cells (left panel) due to the positional loss along the z axis in the strength of the evanescence field.
Fig 7
Outcomes of CPV-TfR interactions identified in this study. CPV capsids (blue) attach to TfRs (orange) on the cell surface. The capsid-receptor complex diffuses laterally on the cell surface, and at times, capsids engage more than one receptor. Receptor-bound capsids can diffuse into an assembling clathrin-coated pit (green, AP-2; red, clathrin), leading to particle endocytosis upon pit scission by dynamin (purple) (outcome 1). Alternatively, capsids can detach from TfRs prior to association with a coated pit (outcome 2).
Similar articles
- Purified feline and canine transferrin receptors reveal complex interactions with the capsids of canine and feline parvoviruses that correspond to their host ranges.
Palermo LM, Hafenstein SL, Parrish CR. Palermo LM, et al. J Virol. 2006 Sep;80(17):8482-92. doi: 10.1128/JVI.00683-06. J Virol. 2006. PMID: 16912298 Free PMC article. - Complex and Dynamic Interactions between Parvovirus Capsids, Transferrin Receptors, and Antibodies Control Cell Infection and Host Range.
Callaway HM, Welsch K, Weichert W, Allison AB, Hafenstein SL, Huang K, Iketani S, Parrish CR. Callaway HM, et al. J Virol. 2018 Jun 13;92(13):e00460-18. doi: 10.1128/JVI.00460-18. Print 2018 Jul 1. J Virol. 2018. PMID: 29695427 Free PMC article. - Canine and feline parvoviruses can use human or feline transferrin receptors to bind, enter, and infect cells.
Parker JS, Murphy WJ, Wang D, O'Brien SJ, Parrish CR. Parker JS, et al. J Virol. 2001 Apr;75(8):3896-902. doi: 10.1128/JVI.75.8.3896-3902.2001. J Virol. 2001. PMID: 11264378 Free PMC article. - [Evolution and host variation of the canine parvovirus: molecular basis for the development of a new virus].
Hueffer K, Truyen U, Parrish CR. Hueffer K, et al. Berl Munch Tierarztl Wochenschr. 2004 Mar-Apr;117(3-4):130-5. Berl Munch Tierarztl Wochenschr. 2004. PMID: 15046459 Review. German. - Receptor-mediated endocytosis: the intracellular journey of transferrin and its receptor.
Dautry-Varsat A. Dautry-Varsat A. Biochimie. 1986 Mar;68(3):375-81. doi: 10.1016/s0300-9084(86)80004-9. Biochimie. 1986. PMID: 2874839 Review.
Cited by
- Transferrin receptor 1 is a supplementary receptor that assists transmissible gastroenteritis virus entry into porcine intestinal epithelium.
Zhang S, Hu W, Yuan L, Yang Q. Zhang S, et al. Cell Commun Signal. 2018 Oct 20;16(1):69. doi: 10.1186/s12964-018-0283-5. Cell Commun Signal. 2018. PMID: 30342530 Free PMC article. - Similar uptake but different trafficking and escape routes of reovirus virions and infectious subvirion particles imaged in polarized Madin-Darby canine kidney cells.
Boulant S, Stanifer M, Kural C, Cureton DK, Massol R, Nibert ML, Kirchhausen T. Boulant S, et al. Mol Biol Cell. 2013 Apr;24(8):1196-207. doi: 10.1091/mbc.E12-12-0852. Epub 2013 Feb 20. Mol Biol Cell. 2013. PMID: 23427267 Free PMC article. - Dynamics of virus-receptor interactions in virus binding, signaling, and endocytosis.
Boulant S, Stanifer M, Lozach PY. Boulant S, et al. Viruses. 2015 Jun 2;7(6):2794-815. doi: 10.3390/v7062747. Viruses. 2015. PMID: 26043381 Free PMC article. Review. - Peptide and protein nanoparticle conjugates: versatile platforms for biomedical applications.
Spicer CD , Jumeaux C , Gupta B , Stevens MM . Spicer CD , et al. Chem Soc Rev. 2018 May 21;47(10):3574-3620. doi: 10.1039/c7cs00877e. Chem Soc Rev. 2018. PMID: 29479622 Free PMC article. Review. - Virus tracking technologies and their applications in viral life cycle: research advances and future perspectives.
Liu D, Pan L, Zhai H, Qiu HJ, Sun Y. Liu D, et al. Front Immunol. 2023 Jun 2;14:1204730. doi: 10.3389/fimmu.2023.1204730. eCollection 2023. Front Immunol. 2023. PMID: 37334362 Free PMC article. Review.
References
- Agbandje M, McKenna R, Rossmann MG, Strassheim ML, Parrish CR. 1993. Structure determination of feline panleukopenia virus empty particles. Proteins 16:155–171 - PubMed
- Cheng Y, Zak O, Aisen P, Harrison SC, Walz T. 2004. Structure of the human transferrin receptor-transferrin complex. Cell 116:565–576 - PubMed
- Collawn JF, et al. 1993. YTRF is the conserved internalization signal of the transferrin receptor, and a second YTRF signal at position 31–34 enhances endocytosis. J. Biol. Chem. 268:21686–21692 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI028385/AI/NIAID NIH HHS/United States
- R01 AI092571/AI/NIAID NIH HHS/United States
- U54 AI057159/AI/NIAID NIH HHS/United States
- AI028385/AI/NIAID NIH HHS/United States
- AI092571/AI/NIAID NIH HHS/United States
- R01 GM075252/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources