Novel, real-time cell analysis for measuring viral cytopathogenesis and the efficacy of neutralizing antibodies to the 2009 influenza A (H1N1) virus - PubMed (original) (raw)
Clinical Trial
Novel, real-time cell analysis for measuring viral cytopathogenesis and the efficacy of neutralizing antibodies to the 2009 influenza A (H1N1) virus
Di Tian et al. PLoS One. 2012.
Erratum in
- PLoS One. 2012;7(7): doi/10.1371/annotation/9f718228-cb10-444b-882b-5dc795e9d1e7
Abstract
A novel electronic cell sensor array technology, the real-time cell analysis (RTCA) system, was developed to monitor cell events. Unlike the conventional methods labeling the target cells with fluorescence, luminescence, or light absorption, the RTCA system allows for label-free detection of cell processes directly without the incorporation of labels. Here, we used this new format to measure the cytopathic effect (CPE) of the 2009 influenza A (H1N1) virus and the efficacy of neutralizing antibodies in human sera to this virus. The real-time dynamic monitoring of CPE was performed on MDCK cell cultures infected with the H1N1 virus, ranging from 5.50×10(2) to 5.50×10(7) copies/mL. The resulting CPE kinetic curves were automatically recorded and were both time and viral load dependent. The CPE kinetics were also distinguishable between different H1N1 stains, as the onset of CPE induced by the A/Shanghai/37T/2009 H1N1 virus was earlier than that of the A/Shanghai/143T/2009 H1N1 virus. Furthermore, inhibition of H1N1 virus-induced CPE in the presence of human specific anti-sera was detected and quantified using the RTCA system. Antibody titers determined using this new neutralization test correlated well with those obtained independently via the standard hemagglutination inhibition test. Taken together, this new CPE assay format provided label-free and high-throughput measurement of viral growth and the effect of neutralizing antibodies, illustrating its potential in influenza vaccine studies.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
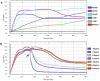
Figure 1. Dynamic monitoring of MDCK cell proliferation.
MDCK cells were seeded in the E-Plates and continuously monitored by measuring the CI to identify the appropriate cell numbers, TPCK-trypsin concentration, and the time point for addition of virus (i.e., during growth or early stationary phase). A) Proliferation curves of MDCK cells in cell culture media. B) Proliferation curves of MDCK cells in SFM with TPCK-trypsin. At the indicated time point the cells were inoculated with different concentrations of virus and TPCK-trypsin.
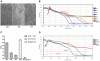
Figure 2. Real-time monitoring of pH1N1 virus-mediated cytopathogenicity.
A) The CPE on MDCK cells infected with SH37T (1∶10 diluted) at 24, 48, and 72 hours post-infection. B) Real-time monitoring of the CPE on MDCK cells infected with SH37T using the RTCA system. The curve was an average of four independent replicate wells. At the indicated time point the cells were inoculated with different dilutions of virus. C) The colorimetric assays for the CPE on MDCK cells infected with SH37T (1∶10 diluted). OD values at A450 obtained at 24, 48, and 72 hours post-infection are shown. Error bars indicate standard deviation. D) Difference in CPE kinetic patterns between SH37T and SH143T with the same infectious vial load of 5.50×107 copies/mL. The plotted lines indicate the different time points when the CI declined to 0 between stains.
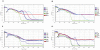
Figure 3. Typical results of real-time neutralization tests against SH37T.
CPE kinetic patterns of MDCK cells inoculated with 100 TCID50 virus incubated with a series dilution of sera samples. Antibody titers were calculated as a function of CI with a sequential dilution series of S0 (A), S1 (B), and S2 (C). The curve was an average of three independent replicate wells. At the indicated time point the cells were inoculated with the serial serum-virus mixture. Three replicate wells of 1280-fold diluted S2 treated cells showed different curves (D). Cell control: MDCK cells only; virus control: absence of test sera.
Figure 4. Antibody titers in 21 pre- and post-vaccination sera triples measured by real-time NT and HI tests.
A, B) There was a statistically significant difference (p<0.01, Wilcoxon signed rank test) when the pre- and post-vaccination titers obtained by real-time NT (A) or HI test (B) were compared to each other. C) The NT and HI antibody titers correlated well (Spearman's correlation _r_ = 0.78, _p_<0.01). D) Antibody titers on day 21 after vaccination relative to baseline (day 0). There was no significant difference (_p_>0.05, Wilcoxon signed rank test) in the results among the NT versus HI tests. Low responders: no increase above twofold; high responders: fourfold or more above baseline.
Similar articles
- Antibodies against swine influenza virus neutralize the pandemic influenza virus A/H1N1.
Tsukamoto M, Hiroi S, Adachi K, Kato H, Inai M, Konishi I, Tanaka M, Yamamoto R, Sawa M, Handharyani E, Tsukamoto Y. Tsukamoto M, et al. Mol Med Rep. 2011 Mar-Apr;4(2):209-14. doi: 10.3892/mmr.2011.410. Epub 2011 Jan 3. Mol Med Rep. 2011. PMID: 21468553 - Association between Hemagglutinin Stem-Reactive Antibodies and Influenza A/H1N1 Virus Infection during the 2009 Pandemic.
Hoa LNM, Mai LQ, Bryant JE, Thai PQ, Hang NLK, Yen NTT, Duong TN, Thoang DD, Horby P, Werheim HFL, Fox A. Hoa LNM, et al. J Virol. 2016 Jun 24;90(14):6549-6556. doi: 10.1128/JVI.00093-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27170747 Free PMC article. - Seasonal H1N1 2007 influenza virus infection is associated with elevated pre-exposure antibody titers to the 2009 pandemic influenza A (H1N1) virus.
Lemaitre M, Leruez-Ville M, De Lamballerie XN, Salez N, Garrone P, Fluckiger AC, Klatzmann D, Carrat F. Lemaitre M, et al. Clin Microbiol Infect. 2011 May;17(5):732-7. doi: 10.1111/j.1469-0691.2010.03352.x. Epub 2010 Oct 29. Clin Microbiol Infect. 2011. PMID: 20731679 - Serum strain-specific or cross-reactive neuraminidase inhibiting antibodies against pandemic А/California/07/2009(H1N1) influenza in healthy volunteers.
Desheva YA, Smolonogina TA, Donina SA, Rudenko LG. Desheva YA, et al. BMC Res Notes. 2015 Apr 10;8:136. doi: 10.1186/s13104-015-1086-z. BMC Res Notes. 2015. PMID: 25889924 Free PMC article. - Pre-vaccination immunity and immune responses to a cell culture-derived whole-virus H1N1 vaccine are similar to a seasonal influenza vaccine.
Ehrlich HJ, Müller M, Kollaritsch H, Pinl F, Schmitt B, Zeitlinger M, Loew-Baselli A, Kreil TR, Kistner O, Portsmouth D, Fritsch S, Maritsch F, Aichinger G, Pavlova BG, Barrett PN. Ehrlich HJ, et al. Vaccine. 2012 Jun 22;30(30):4543-51. doi: 10.1016/j.vaccine.2012.03.061. Epub 2012 Apr 1. Vaccine. 2012. PMID: 22475864 Clinical Trial.
Cited by
- Application of a real-time cell analysis system in the process development and quantification of Rift Valley fever virus clone 13.
Moetlhoa B, Naicker L, Hayeshi R, Grobler A, Mokoena NB, Mawadza C. Moetlhoa B, et al. Access Microbiol. 2020 Dec 17;3(3):000191. doi: 10.1099/acmi.0.000191. eCollection 2021 Mar. Access Microbiol. 2020. PMID: 34151150 Free PMC article. - Real-time cell analysis for monitoring cholera toxin-induced human intestinal epithelial cell response.
Ye J, Luo Y, Fang W, Pan J, Zhang Z, Zhang Y, Chen Z, Jin D. Ye J, et al. Curr Microbiol. 2015 Apr;70(4):536-43. doi: 10.1007/s00284-014-0752-z. Epub 2014 Dec 16. Curr Microbiol. 2015. PMID: 25510171 - Effects for Sequential Treatment of siAkt and Paclitaxel on Gastric Cancer Cell Lines.
Ku M, Kang M, Suh JS, Yang J. Ku M, et al. Int J Med Sci. 2016 Sep 7;13(9):708-16. doi: 10.7150/ijms.15501. eCollection 2016. Int J Med Sci. 2016. PMID: 27648001 Free PMC article. - Role of microRNA 30a targeting insulin receptor substrate 2 in colorectal tumorigenesis.
Zhang Q, Tang Q, Qin D, Yu L, Huang R, Lv G, Zou Z, Jiang XC, Zou C, Liu W, Luo J, Zhao Z, Muhammad S, Wang G, Chen YG, Wang X. Zhang Q, et al. Mol Cell Biol. 2015 Mar;35(6):988-1000. doi: 10.1128/MCB.01242-14. Epub 2015 Jan 12. Mol Cell Biol. 2015. PMID: 25582198 Free PMC article. Retracted. - Development of a Real-Time Cell Analysis (RTCA) Method as a Fast and Accurate Method for Detecting Infectious Particles of the Adapted Strain of Hepatitis A Virus.
Lebourgeois S, Fraisse A, Hennechart-Collette C, Guillier L, Perelle S, Martin-Latil S. Lebourgeois S, et al. Front Cell Infect Microbiol. 2018 Sep 25;8:335. doi: 10.3389/fcimb.2018.00335. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30319992 Free PMC article.
References
- Zhu FC, Wang H, Fang HH, Yang JG, Lin XJ, et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med. 2009;361:2414–2423. - PubMed
- Jiang W, Xu F, Lu L, Lu M, Miao L, et al. Safety and effectiveness of a 2009 H1N1 vaccine in Beijing. N Engl J Med. 2010;363:2416–2423. - PubMed
- Liang XF, Wang HQ, Wang JZ, Fang HH, Wu J, et al. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2010;375:56–66. - PubMed
- Smee DF, Morrison AC, Barnard DL, Sidwell RW. Comparison of colorimetric, fluorometric, and visual methods for determining anti-influenza (H1N1 and H3N2) virus activities and toxicities of compounds. J Virol Methods. 2002;106:71–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous