Beam-induced motion of vitrified specimen on holey carbon film - PubMed (original) (raw)
Beam-induced motion of vitrified specimen on holey carbon film
Axel F Brilot et al. J Struct Biol. 2012 Mar.
Abstract
The contrast observed in images of frozen-hydrated biological specimens prepared for electron cryo-microscopy falls significantly short of theoretical predictions. In addition to limits imposed by the current instrumentation, it is widely acknowledged that motion of the specimen during its exposure to the electron beam leads to significant blurring in the recorded images. We have studied the amount and direction of motion of virus particles suspended in thin vitrified ice layers across holes in perforated carbon films using exposure series. Our data show that the particle motion is correlated within patches of 0.3-0.5 μm, indicating that the whole ice layer is moving in a drum-like motion, with accompanying particle rotations of up to a few degrees. Support films with smaller holes, as well as lower electron dose rates tend to reduce beam-induced specimen motion, consistent with a mechanical effect. Finally, analysis of movies showing changes in the specimen during beam exposure show that the specimen moves significantly more at the start of an exposure than towards its end. We show how alignment and averaging of movie frames can be used to restore high-resolution detail in images affected by beam-induced motion.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Figure 1
Exposure series of rotavirus DLPs in ice in a C-flat™ 1.2/1.3 grid (hole size = 1.2 μm). Four successive exposures are shown in panels A, B, C and D. The total dose applied to the sample is noted above each exposure. Panels E, F and G show vector plots corresponding to the changes in particle orientations between exposures (exposure numbers are indicated in the plots). Panel H plots the summed vectors from panels E, F and G.
Figure 2
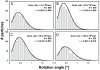
Histograms for particle rotations measured between the first and second exposures of rotavirus DLPs in ice on C-flat™ 1.2/1.3 Cu 400 mesh grids (hole size = 1.2 μm). The dose applied in each exposure was 8 electrons/Å2. The dose rate varied between 2 and 160 electrons/Å2/sec, giving exposure times between 0.05 – 4 sec. The histograms were fitted with a Rayleigh distribution and maximum (λ) is indicated in each plot together with the total number of measurements.
Figure 3
Movie frame averages of rotavirus DLPs in ice on a Quantifoil® grid (hole size = 1.6 μm). The movie was recorded for 1.5 sec at 40 frames/sec using the DE-12 direct electron detector (Direct Electron). 10-frame averages of the resulting 60 frames were subjected to the same analysis as the exposure series. Vector plots in panels A – E indicate particle rotations measured between frame averages. The total average of the movie is shown in the background in A and the summed rotations from panels A – E are shown in F. Panels G – K show vector plots indicating the particle translations measured between frame averages. Panel L shows the summed translations from panels G – K. The square highlights the area shown as a close-up in Figure 5. The movie is further analyzed in Supplementary material (Movies S1 – S3).
Figure 4
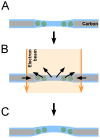
Schematic of beam-induced motion. A) Particles are suspended in vitrified ice over a hole. B) When the electron beam illuminates the sample, the carbon support film deforms such that the size of the hole shrinks by a small amount. At the same time, radiolysis of the solvent and macromolecules produces radicals that increase the pressure inside the ice layer. Both changes cause a drum motion of the ice layer that lead to particle rotations and translations. The direction of the drum motion is random. C) After the beam is turned off, the changes induced in the specimen remain (plastic deformation). This model does not consider specimen charging which could add to the blurring of images.
Figure 5
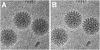
Translational alignment of movie frames to reduce blurring in images affected by beam-induced motion. A) Average of 60 frames of an area of Movie S1 that experienced translations of about 60 Å (see Figure 3L) Particles are significantly blurred and high-resolution information is lost. B) Average of the same 60 frames as in panel A after translational alignment of individual frames. The translations for the individual frames were calculated from the translations determined in Figure 3 for the 10-frame averages by linear interpolation. The frame average in panel B exhibits substantially improved contrast with details at higher resolution that were not visible in panel A.
Similar articles
- Non-rigid image registration to reduce beam-induced blurring of cryo-electron microscopy images.
Karimi Nejadasl F, Karuppasamy M, Newman ER, McGeehan JE, Ravelli RB. Karimi Nejadasl F, et al. J Synchrotron Radiat. 2013 Jan;20(Pt 1):58-66. doi: 10.1107/S0909049512044408. Epub 2012 Nov 29. J Synchrotron Radiat. 2013. PMID: 23254656 Free PMC article. - Movies of ice-embedded particles enhance resolution in electron cryo-microscopy.
Campbell MG, Cheng A, Brilot AF, Moeller A, Lyumkis D, Veesler D, Pan J, Harrison SC, Potter CS, Carragher B, Grigorieff N. Campbell MG, et al. Structure. 2012 Nov 7;20(11):1823-8. doi: 10.1016/j.str.2012.08.026. Epub 2012 Sep 27. Structure. 2012. PMID: 23022349 Free PMC article. - Fabrication of carbon films with ∼ 500nm holes for cryo-EM with a direct detector device.
Marr CR, Benlekbir S, Rubinstein JL. Marr CR, et al. J Struct Biol. 2014 Jan;185(1):42-7. doi: 10.1016/j.jsb.2013.11.002. Epub 2013 Nov 21. J Struct Biol. 2014. PMID: 24269484 - Specimen Behavior in the Electron Beam.
Glaeser RM. Glaeser RM. Methods Enzymol. 2016;579:19-50. doi: 10.1016/bs.mie.2016.04.010. Epub 2016 May 31. Methods Enzymol. 2016. PMID: 27572722 Review. - Processing of Cryo-EM Movie Data.
Ripstein ZA, Rubinstein JL. Ripstein ZA, et al. Methods Enzymol. 2016;579:103-24. doi: 10.1016/bs.mie.2016.04.009. Epub 2016 Jun 1. Methods Enzymol. 2016. PMID: 27572725 Review.
Cited by
- Single-Particle Cryo-EM at Crystallographic Resolution.
Cheng Y. Cheng Y. Cell. 2015 Apr 23;161(3):450-457. doi: 10.1016/j.cell.2015.03.049. Cell. 2015. PMID: 25910205 Free PMC article. Review. - 2.8 Å resolution reconstruction of the Thermoplasma acidophilum 20S proteasome using cryo-electron microscopy.
Campbell MG, Veesler D, Cheng A, Potter CS, Carragher B. Campbell MG, et al. Elife. 2015 Mar 11;4:e06380. doi: 10.7554/eLife.06380. Elife. 2015. PMID: 25760083 Free PMC article. - Cryo-EM structure of a CD4-bound open HIV-1 envelope trimer reveals structural rearrangements of the gp120 V1V2 loop.
Wang H, Cohen AA, Galimidi RP, Gristick HB, Jensen GJ, Bjorkman PJ. Wang H, et al. Proc Natl Acad Sci U S A. 2016 Nov 15;113(46):E7151-E7158. doi: 10.1073/pnas.1615939113. Epub 2016 Oct 31. Proc Natl Acad Sci U S A. 2016. PMID: 27799557 Free PMC article. - Exploring the Structural Variability of Dynamic Biological Complexes by Single-Particle Cryo-Electron Microscopy.
DiIorio MC, Kulczyk AW. DiIorio MC, et al. Micromachines (Basel). 2022 Dec 31;14(1):118. doi: 10.3390/mi14010118. Micromachines (Basel). 2022. PMID: 36677177 Free PMC article. Review. - Phase contrast imaging with inelastically scattered electrons from any layer of a thick specimen.
Dickerson JL, Russo CJ. Dickerson JL, et al. Ultramicroscopy. 2022 Jul;237:113511. doi: 10.1016/j.ultramic.2022.113511. Epub 2022 Mar 19. Ultramicroscopy. 2022. PMID: 35367902 Free PMC article.
References
- Baker LA, Smith EA, Bueler SA, Rubinstein JL. The resolution dependence of optimal exposures in liquid nitrogen temperature electron cryomicroscopy of catalase crystals. Journal of structural biology. 2010;169:431–437. - PubMed
- Booy FP, Pawley JB. Cryo-crinkling: what happens to carbon films on copper grids at low temperature. Ultramicroscopy. 1993;48:273–280. - PubMed
- Bottcher B. Electron cryomicroscopy of graphite in amorphous Ice. Ultramicroscopy. 1995;58:417–424.
- Brink J, Sherman MB, Berriman J, Chiu W. Evaluation of charging on macromolecules in electron cryomicroscopy. Ultramicroscopy. 1998;72:41–52. - PubMed
- Bullough P, Henderson R. Use of spot-scan procedure for recording low-dose micrographs of beam-sensitive specimens. Ultramicroscopy. 1987;21:223–229.
Publication types
MeSH terms
Grants and funding
- P01 GM062580/GM/NIGMS NIH HHS/United States
- MC_U105184322/MRC_/Medical Research Council/United Kingdom
- P01 GM62580/GM/NIGMS NIH HHS/United States
- P41 RR017573/RR/NCRR NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- RR017573/RR/NCRR NIH HHS/United States
- P01 GM062580-10/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources