Type VI secretion requires a dynamic contractile phage tail-like structure - PubMed (original) (raw)
Type VI secretion requires a dynamic contractile phage tail-like structure
M Basler et al. Nature. 2012.
Abstract
Type VI secretion systems are bacterial virulence-associated nanomachines composed of proteins that are evolutionarily related to components of bacteriophage tails. Here we show that protein secretion by the type VI secretion system of Vibrio cholerae requires the action of a dynamic intracellular tubular structure that is structurally and functionally homologous to contractile phage tail sheath. Time-lapse fluorescence light microscopy reveals that sheaths of the type VI secretion system cycle between assembly, quick contraction, disassembly and re-assembly. Whole-cell electron cryotomography further shows that the sheaths appear as long tubular structures in either extended or contracted conformations that are connected to the inner membrane by a distinct basal structure. These data support a model in which the contraction of the type VI secretion system sheath provides the energy needed to translocate proteins out of effector cells and into adjacent target cells.
Figures
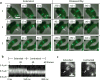
Figure 1. Fluorescence light microscopy of VipA-sfGFP
(a) Individual 3×3 μm frames from a time-lapse imaging with a frame rate of 10 sec per frame show 3 frames of extension of VipA-sfGFP structure in ΔVipA background from one side of the cell to another (arrows) followed by a contraction event and apparent disassembly (shown on 3 frames) of the contracted VipA-sfGFP structure (arrows). Bar shown on the first frame represents 1 μm. The whole 10 minute time-lapse sequence is shown in Supplementary Video 1 together with another 17 similar events, larger field of cells is shown in Supplementary Video 2. (b) Kymogram illustrating rapid change in the length of VipA-sfGFP structure. Projection of signal intensity in time at a rate of 200 frames per second along the axis of the maximal intensity on an extended structure (30 frame average shown on the panel (c) left) showing a contraction in length and increase in maximal intensity of the contracted structure (30 frame average shown on the panel (c) right). Arrows indicate contracting VipA-sfGFP structure and mark start and end of a line for generating the kymogram. Bar shown on the average frames is 1 μm long. Gaussian blur filter (sigma radius = 1) was applied to individual frames prior to generating the kymogram. All 60 frames of the time-lapse sequence are shown in Supplementary Video 3 (video segment number 3) together with 4 more contraction events imaged at the same or lower frame rate.
Figure 2. Electron cryotomographic imaging of T6SS structures inside intact cells
Shown are different tomographic slices (19 nm in a, e, c, g; 9.5 nm in b,f; 190 nm in d, h) of an extended (a–d) and a contracted (e–h) structure imaged in two different wild type cells (contracted/extended structures, T6SS; IM, inner membrane; OM, outer membrane; F, flagellum; R, putative ribosome; SG, polyphosphate storage granule). (b) and (f), each show three slices at the same orientation but at different Z-heights. Compared to extended structures, contracted structures are shorter (b, f), have a helical surface pattern (pitch angle of 87°) and a smaller diameter (indicated in the perpendicular views in d, h). (c) and (g) are segmentations of densities observed in the extended (c) and contracted (g) structures. Densities shown in (h) originate from a contracted structure from a different tomogram. Segmented are putative densities corresponding to sheath (green), baseplate (pink and yellow) and membranes (blue). Bar in (a) 100 nm (applies to a, e), bar in (b) 100 nm (applies to b, f), bar in (c) 20 nm (applies to c, d, g, h).
Figure 3. Images of purified VipA/VipB sheaths and comparison with phage tails
Negative staining (a) images of purified wild type sheath (left) and VipA-sfGFP-labeled sheath (right) are highly similar except for flared extra densities on the outside of the VipA-sfGFP-labeled structure. Cryotomograms of wild type sheath (b, shown three 12.6-nm slices at different Z-heights) were highly similar to contracted structures imaged in vivo (Figure 2f). Note the matching surface pitch angle of 87° seen in tomographic slices (b) and an isosurface of a subtomogram average (c). The negatively stained perpendicular view of a purified wild type sheath showed the characteristic “cog-wheel” like structure with 12 paddles (d) and is similar to the perpendicular view of a contracted T4 phage sheath (e, left; two rings of six gp18 subunits, created in Chimera from EMDB 1086 map). Similar to T6SS sheath (c), also the surface of the contracted T4 phage sheath appears helical (e, right) though with a different pitch angle. Bar in (b) 20 nm (applies to a,b), bar in (e) 10 nm (applies to c–e). Note that protein densities appear white in negative stain images and black in cryotomograms.
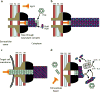
Figure 4. Model of T6SS action
IM – inner membrane, PG – peptidoglycan, OM – outer membrane. (a) Assembly - First step is a base plate complex formation that initiates the Hcp tube polymerization. The base plate complex is likely composed of gp25, VgrG and other T6SS proteins that define a bell-shaped cytoplasmic component (black objects) and periplasmic component (brown objects) which together span the inner membrane, peptidoglycan, and outer membrane. Second step is polymerization of the sheath (from VipA/VipB heterodimers) around the Hcp tube in an extended conformation. (b) Extended T6SS apparatus in extended “ready to fire” conformation. The membrane distal end may be capped by an unknown protein or VipAB conformational state. (c) Contraction - Upon an unknown extracellular signal a conformational change in the base plate complex triggers sheath contraction that leads to the translocation (secretion) of the VgrG/Hcp tube complex through effector cell membranes and penetration of adjacent target cell membrane. Translocation of additional effector proteins might then follow using the Hcp tube as a conduit. (d) Disassembly - Contracted sheath is detached and disassembled by ClpV ATPase. VipA/B dimers released are recycled into a new extended T6SS apparatus at either the original or a newly formed base plate complex. In the absence of target cell penetration (see panel c), Hcp and VgrG proteins are released into the extracellular space as secreted proteins.
Comment in
- Bacterial secretion: Highly sprung secretion.
Jermy A. Jermy A. Nat Rev Microbiol. 2012 Mar 16;10(4):238. doi: 10.1038/nrmicro2775. Nat Rev Microbiol. 2012. PMID: 22421876 No abstract available.
Similar articles
- Structure of the type VI secretion system contractile sheath.
Kudryashev M, Wang RY, Brackmann M, Scherer S, Maier T, Baker D, DiMaio F, Stahlberg H, Egelman EH, Basler M. Kudryashev M, et al. Cell. 2015 Feb 26;160(5):952-962. doi: 10.1016/j.cell.2015.01.037. Cell. 2015. PMID: 25723169 Free PMC article. - Atomic structure of T6SS reveals interlaced array essential to function.
Clemens DL, Ge P, Lee BY, Horwitz MA, Zhou ZH. Clemens DL, et al. Cell. 2015 Feb 26;160(5):940-951. doi: 10.1016/j.cell.2015.02.005. Cell. 2015. PMID: 25723168 Free PMC article. - Cryo-EM reconstruction of Type VI secretion system baseplate and sheath distal end.
Nazarov S, Schneider JP, Brackmann M, Goldie KN, Stahlberg H, Basler M. Nazarov S, et al. EMBO J. 2018 Feb 15;37(4):e97103. doi: 10.15252/embj.201797103. Epub 2017 Dec 18. EMBO J. 2018. PMID: 29255010 Free PMC article. - Architecture and assembly of the Type VI secretion system.
Zoued A, Brunet YR, Durand E, Aschtgen MS, Logger L, Douzi B, Journet L, Cambillau C, Cascales E. Zoued A, et al. Biochim Biophys Acta. 2014 Aug;1843(8):1664-73. doi: 10.1016/j.bbamcr.2014.03.018. Epub 2014 Mar 26. Biochim Biophys Acta. 2014. PMID: 24681160 Review. - VgrG, Tae, Tle, and beyond: the versatile arsenal of Type VI secretion effectors.
Durand E, Cambillau C, Cascales E, Journet L. Durand E, et al. Trends Microbiol. 2014 Sep;22(9):498-507. doi: 10.1016/j.tim.2014.06.004. Epub 2014 Jul 17. Trends Microbiol. 2014. PMID: 25042941 Review.
Cited by
- Evaluation of the roles played by Hcp and VgrG type 6 secretion system effectors in Aeromonas hydrophila SSU pathogenesis.
Sha J, Rosenzweig JA, Kozlova EV, Wang S, Erova TE, Kirtley ML, van Lier CJ, Chopra AK. Sha J, et al. Microbiology (Reading). 2013 Jun;159(Pt 6):1120-1135. doi: 10.1099/mic.0.063495-0. Epub 2013 Mar 21. Microbiology (Reading). 2013. PMID: 23519162 Free PMC article. - Secretion systems in Gram-negative bacteria: structural and mechanistic insights.
Costa TR, Felisberto-Rodrigues C, Meir A, Prevost MS, Redzej A, Trokter M, Waksman G. Costa TR, et al. Nat Rev Microbiol. 2015 Jun;13(6):343-59. doi: 10.1038/nrmicro3456. Nat Rev Microbiol. 2015. PMID: 25978706 Review. - Structure of a peptidoglycan amidase effector targeted to Gram-negative bacteria by the type VI secretion system.
Chou S, Bui NK, Russell AB, Lexa KW, Gardiner TE, LeRoux M, Vollmer W, Mougous JD. Chou S, et al. Cell Rep. 2012 Jun 28;1(6):656-64. doi: 10.1016/j.celrep.2012.05.016. Epub 2012 May 31. Cell Rep. 2012. PMID: 22813741 Free PMC article. - Bacterial symbionts use a type VI secretion system to eliminate competitors in their natural host.
Speare L, Cecere AG, Guckes KR, Smith S, Wollenberg MS, Mandel MJ, Miyashiro T, Septer AN. Speare L, et al. Proc Natl Acad Sci U S A. 2018 Sep 4;115(36):E8528-E8537. doi: 10.1073/pnas.1808302115. Epub 2018 Aug 20. Proc Natl Acad Sci U S A. 2018. PMID: 30127013 Free PMC article. - R-Type Fonticins Produced by Pragia fontium Form Large Pores with High Conductance.
Látrová K, Dolejšová T, Motlová L, Mikušová G, Bosák J, Snopková K, Šmajs D, Konopásek I, Fišer R. Látrová K, et al. J Bacteriol. 2023 Jan 26;205(1):e0031522. doi: 10.1128/jb.00315-22. Epub 2022 Dec 21. J Bacteriol. 2023. PMID: 36541812 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI026289/AI/NIAID NIH HHS/United States
- R01 AI018045/AI/NIAID NIH HHS/United States
- AI-26289/AI/NIAID NIH HHS/United States
- R01 GM094800/GM/NIGMS NIH HHS/United States
- AI-018045/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R37 AI018045/AI/NIAID NIH HHS/United States
- GM094800B/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases