Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer's disease neurons: implications for mitochondrial dysfunction and neuronal damage - PubMed (original) (raw)
. 2012 Jun 1;21(11):2538-47.
doi: 10.1093/hmg/dds072. Epub 2012 Feb 24.
Affiliations
- PMID: 22367970
- PMCID: PMC3349426
- DOI: 10.1093/hmg/dds072
Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer's disease neurons: implications for mitochondrial dysfunction and neuronal damage
Maria Manczak et al. Hum Mol Genet. 2012.
Abstract
We recently reported increased mitochondrial fission and decreased fusion, increased amyloid beta (Aβ) interaction with the mitochondrial fission protein Drp1, increased mitochondrial fragmentation, impaired axonal transport of mitochondria and synaptic degeneration in neurons affected by AD. In the present study, we extended our previous investigations to determine whether phosphorylated tau interacts with Drp1 and to elucidate mitochondrial damage in the progression of AD. We also investigated GTPase activity, which is critical for mitochondrial fragmentation, in postmortem brain tissues from patients with AD and brain tissues from APP, APP/PS1 and 3XTg.AD mice. Using co-immunoprecipitation and immunofluorescence analyses, for the first time, we demonstrated the physical interaction between phosphorylated tau and Drp1. Mitochondrial fission-linked GTPase activity was significantly elevated in the postmortem frontal cortex tissues from AD patients and cortical tissues from APP, APP/PS1 and 3XTg.AD mice. On the basis of these findings, we conclude that Drp1 interacts with Aβ and phosphorylated tau, likely leading to excessive mitochondrial fragmentation, and mitochondrial and synaptic deficiencies, ultimately possibly leading to neuronal damage and cognitive decline. Treatment designed to reduce the expression of Drp1, Aβ and/or phosphorylated tau may decrease the interaction between Drp1 and phosphorylated tau and the interaction between Drp1 and Aβ, conferring protection to neurons from toxic insults of excessive Drp1, Aβ and/or phosphorylated tau.
Figures
Figure 1.
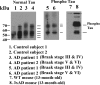
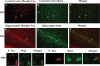
A representative western blot analysis of normal and phosphorylated tau proteins in brain tissues from early (Braak stages I and II, n = 5), definite (Braak stages III and IV, n = 5) and severe (Braak stages V and VI, n = 5) AD patients, control subjects (Braak stage 0, n = 5) and 3XTg.AD mice (n = 5) and WT mice (n = 5). Normal tau is phosphorylated in AD patients and 3XTg.AD mice.
Figure 2.
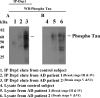
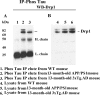
Co-IP analysis of Drp1 and phosphorylated tau in AD patients. (A) Results from IP with a Drp1 antibody and from western blot analysis with a phosphorylated tau antibody. Phosphorylated tau was found in Drp1-IP elutes from AD patients. (B) Western blot analysis of protein lysates from AD patients and control subjects, using the Drp1 antibody.
Figure 3.
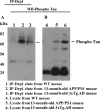
Co-IP analysis of phosphorylated tau and Drp1 in AD patients. (A) IP results with a phosphorylated tau antibody and results from western blot analysis with the Drp1 antibody. Drp1 was found in phosphorylated tau-IP elutes in the cortical tissues from AD patients. (B) Western blots of protein lysates from AD patients and control subjects, using the phosphorylated tau antibody.
Figure 4.
Co-IP analysis of Drp1 and phosphorylated tau in APP/PS1 and 3XTg.AD mice. (A) IP results with the Drp1 antibody and results from western blot analysis with the phosphorylated tau antibody. Phosphorylated tau was found in Drp1-IP elutes in the cortical tissues from AD patients. (B) Western blot analysis of protein lysates from in APP/PS1 and 3XTg.AD mice, using the Drp1 antibody.
Figure 5.
Co-IP analysis of phosphorylated tau and Drp1 in APP/PS1 and 3XTg.AD mice. (A) IP results with the phosphorylated tau antibody and results from western blot analysis with the Drp1 antibody. Drp1 was found in phosphorylated tau-IP elutes, in the cortical tissues from AD patients. (B) Results from western blot analysis of protein lysates from APP/PS1 and 3XTg.AD mice, using phosphorylated tau antibody.
Figure 6.
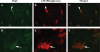
Double-labeling immunofluorescence analysis of phosphorylated tau and Drp1 in AD patients. The localization of (A) Drp1 and (B) phosphorylated tau, and (C, merged) the colocalization of Drp1 and phosphorylated tau at 40× the original magnification. (D) Images of Drp1, (E) phosphorylated tau and (F) merged at 100× the original magnification. Arrows indicate localization of phosphorylated tau, Drp1 and colocalization of both.
Figure 7.
Double-labeling immunofluorescence analysis of phosphorylated tau and Drp1 in 3XTg.AD mice. The localization of (A) Drp1 and (B) phosphorylated tau, and (C, merged) the colocalization of Drp1 and phosphorylated tau in the cortex and hippocampus of AD patients; images taken at 40× the original magnification; images of Drp1 and phosphorylated tau were merged at 100× the original magnification. Arrows indicate localization of phosphorylated tau, Drp1 and colocalization of both.
Figure 8.
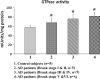
GTPase enzymatic activity in cortical tissues of postmortem brains from AD patients—early AD, Braak stages I and II (n = 5), definite AD, Braak stages III and IV (n = 5), severe AD, Braak stage (n = 5) and control subjects (n = 5). GTPase enzymatic activity was significantly decreased in AD patients relative to control subjects.
Figure 9.
GTPase enzymatic activity in cortical tissues from cortical tissues from the APP (n = 6), APP/PS1 mice (n = 6), 3XTg.AD mice (n = 5) and WT mice (n = 6). GTPase enzymatic activity was significantly increased in the APP, APP/PS1 mice and 3XTg.AD mice relative to WT, non-transgenic mice.
Similar articles
- Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: implications for neuronal damage.
Manczak M, Calkins MJ, Reddy PH. Manczak M, et al. Hum Mol Genet. 2011 Jul 1;20(13):2495-509. doi: 10.1093/hmg/ddr139. Epub 2011 Mar 31. Hum Mol Genet. 2011. PMID: 21459773 Free PMC article. - Abnormal interaction of VDAC1 with amyloid beta and phosphorylated tau causes mitochondrial dysfunction in Alzheimer's disease.
Manczak M, Reddy PH. Manczak M, et al. Hum Mol Genet. 2012 Dec 1;21(23):5131-46. doi: 10.1093/hmg/dds360. Epub 2012 Aug 27. Hum Mol Genet. 2012. PMID: 22926141 Free PMC article. - Reduced dynamin-related protein 1 protects against phosphorylated Tau-induced mitochondrial dysfunction and synaptic damage in Alzheimer's disease.
Kandimalla R, Manczak M, Fry D, Suneetha Y, Sesaki H, Reddy PH. Kandimalla R, et al. Hum Mol Genet. 2016 Nov 15;25(22):4881-4897. doi: 10.1093/hmg/ddw312. Hum Mol Genet. 2016. PMID: 28173111 Free PMC article. - Multiple faces of dynamin-related protein 1 and its role in Alzheimer's disease pathogenesis.
Kandimalla R, Reddy PH. Kandimalla R, et al. Biochim Biophys Acta. 2016 Apr;1862(4):814-828. doi: 10.1016/j.bbadis.2015.12.018. Epub 2015 Dec 17. Biochim Biophys Acta. 2016. PMID: 26708942 Free PMC article. Review. - Amyloid Beta and Phosphorylated Tau-Induced Defective Autophagy and Mitophagy in Alzheimer's Disease.
Reddy PH, Oliver DM. Reddy PH, et al. Cells. 2019 May 22;8(5):488. doi: 10.3390/cells8050488. Cells. 2019. PMID: 31121890 Free PMC article. Review.
Cited by
- Multi-omic profiling reveals the ataxia protein sacsin is required for integrin trafficking and synaptic organization.
Romano LEL, Aw WY, Hixson KM, Novoselova TV, Havener TM, Howell S, Taylor-Blake B, Hall CL, Xing L, Beri J, Nethisinghe S, Perna L, Hatimy A, Altadonna GC, Graves LM, Herring LE, Hickey AJ, Thalassinos K, Chapple JP, Wolter JM. Romano LEL, et al. Cell Rep. 2022 Nov 1;41(5):111580. doi: 10.1016/j.celrep.2022.111580. Cell Rep. 2022. PMID: 36323248 Free PMC article. - Battling Alzheimer's Disease: Targeting SUMOylation-Mediated Pathways.
Martins WC, Tasca CI, Cimarosti H. Martins WC, et al. Neurochem Res. 2016 Mar;41(3):568-78. doi: 10.1007/s11064-015-1681-3. Epub 2015 Jul 31. Neurochem Res. 2016. PMID: 26227998 Review. - Targeting tau in Alzheimer's disease: from mechanisms to clinical therapy.
Ye J, Wan H, Chen S, Liu GP. Ye J, et al. Neural Regen Res. 2024 Jul 1;19(7):1489-1498. doi: 10.4103/1673-5374.385847. Epub 2023 Sep 22. Neural Regen Res. 2024. PMID: 38051891 Free PMC article. - Exploring the bi-directional relationship between autophagy and Alzheimer's disease.
Kuang H, Tan CY, Tian HZ, Liu LH, Yang MW, Hong FF, Yang SL. Kuang H, et al. CNS Neurosci Ther. 2020 Feb;26(2):155-166. doi: 10.1111/cns.13216. Epub 2019 Sep 10. CNS Neurosci Ther. 2020. PMID: 31503421 Free PMC article. Review. - Molecular Mechanisms of Neuroinflammation in Aging and Alzheimer's Disease Progression.
Andronie-Cioara FL, Ardelean AI, Nistor-Cseppento CD, Jurcau A, Jurcau MC, Pascalau N, Marcu F. Andronie-Cioara FL, et al. Int J Mol Sci. 2023 Jan 18;24(3):1869. doi: 10.3390/ijms24031869. Int J Mol Sci. 2023. PMID: 36768235 Free PMC article. Review.
References
- Mattson M.P. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi:10.1038/nature02621. - DOI - PMC - PubMed
- World Alzheimer Report. 2009.
- Terry R.D., Masliah E., Salmon D.P., Butters N., DeTeresa R., Hill R., Hansen L.A., Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30:572–580. doi:10.1002/ana.410300410. - DOI - PubMed
- Selkoe D.J. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi:10.1126/science.1074069. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous