Carboplatin-associated ototoxicity in children with retinoblastoma - PubMed (original) (raw)
Carboplatin-associated ototoxicity in children with retinoblastoma
Ibrahim Qaddoumi et al. J Clin Oncol. 2012.
Abstract
Purpose: Carboplatin-induced ototoxicity remains poorly defined but is of potential great consequence in children with retinoblastoma. We retrospectively assessed the incidence of ototoxicity and its risk factors in children with retinoblastoma who were treated with carboplatin.
Patients and methods: We reviewed the audiologic test results of 60 patients with retinoblastoma who received front-line treatment with systemic carboplatin and vincristine according to the St Jude RET-3 protocol (n = 23) or best clinical management (n = 37). Ototoxicity was evaluated by three different grading systems.
Results: Twelve patients (20%) developed ototoxicity at some time after treatment initiation; however, ototoxicity resolved in two patients, and thus,10 patients (17%) had sustained hearing loss as documented at their most recent audiologic evaluation. Nine of these 10 patients had grade 3 or 4 ototoxicity, and nine patients were less than 6 months of age at the start of chemotherapy. Age at the start of chemotherapy was the only risk factor identified as a significant predictor of sustained hearing loss. Younger age was associated with an increased incidence of hearing loss. The different ototoxicity grading systems showed good overall agreement in the identification of patients with ototoxicity. Agreement was greatest between the Brock and Children's Cancer Group systems.
Conclusion: We found that young patients with retinoblastoma who were treated with systemic carboplatin had a higher incidence of ototoxicity than previously reported. Younger patients (< 6 months of age at the start of treatment) were more likely to have ototoxicity than were older patients. Children treated with carboplatin should routinely undergo thorough, long-term audiologic monitoring.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
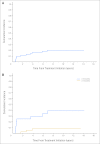
Fig 1.
(A) Cumulative incidence of hearing loss in all patients. (B) Cumulative incidence of hearing loss by age at treatment initiation (< 6 months [n = 24] v ≥ 6 months [n = 37]; P = .004).
Fig 2.
Scatter plot of age at treatment initiation and cumulative carboplatin dose.
Comment in
- A cautionary tale: dosing chemotherapy in infants with retinoblastoma.
Leahey A. Leahey A. J Clin Oncol. 2012 Apr 1;30(10):1023-4. doi: 10.1200/JCO.2011.39.4254. Epub 2012 Feb 27. J Clin Oncol. 2012. PMID: 22370322 No abstract available. - Carboplatin dosing in infants with retinoblastoma: a case for therapeutic drug monitoring.
Veal GJ, Boddy AV. Veal GJ, et al. J Clin Oncol. 2012 Sep 20;30(27):3424; author reply 3425. doi: 10.1200/JCO.2012.43.5677. Epub 2012 Jul 30. J Clin Oncol. 2012. PMID: 22851560 No abstract available.
Similar articles
- A retrospective review of hearing in children with retinoblastoma treated with carboplatin-based chemotherapy.
Lambert MP, Shields C, Meadows AT. Lambert MP, et al. Pediatr Blood Cancer. 2008 Feb;50(2):223-6. doi: 10.1002/pbc.21155. Pediatr Blood Cancer. 2008. PMID: 17278120 - Clinical and genetic associations for carboplatin-related ototoxicity in children treated for retinoblastoma: A retrospective noncomparative single-institute experience.
Soliman SE, D'Silva CN, Dimaras H, Dzneladze I, Chan H, Gallie BL. Soliman SE, et al. Pediatr Blood Cancer. 2018 May;65(5):e26931. doi: 10.1002/pbc.26931. Epub 2018 Jan 19. Pediatr Blood Cancer. 2018. PMID: 29350448 Clinical Trial. - Long-term audiologic follow-up of carboplatin-treated children with retinoblastoma.
Geurtsen ML, Kors WA, Moll AC, Smits C. Geurtsen ML, et al. Ophthalmic Genet. 2017 Jan-Feb;38(1):74-78. doi: 10.3109/13816810.2015.1137325. Epub 2016 Apr 6. Ophthalmic Genet. 2017. PMID: 27050825 - Assessment of hearing in very young children receiving carboplatin for retinoblastoma.
Smits C, Swen SJ, Theo Goverts S, Moll AC, Imhof SM, Schouten-van Meeteren AY. Smits C, et al. Eur J Cancer. 2006 Mar;42(4):492-500. doi: 10.1016/j.ejca.2005.11.004. Epub 2006 Jan 11. Eur J Cancer. 2006. PMID: 16376542 Review. - Ototoxicity monitoring in children treated with platinum chemotherapy.
Brooks B, Knight K. Brooks B, et al. Int J Audiol. 2018 Sep;57(sup4):S34-S40. doi: 10.1080/14992027.2017.1355570. Epub 2017 Jul 24. Int J Audiol. 2018. PMID: 28737048 Review.
Cited by
- Identification of cisplatin-binding proteins using agarose conjugates of platinum compounds.
Karasawa T, Sibrian-Vazquez M, Strongin RM, Steyger PS. Karasawa T, et al. PLoS One. 2013 Jun 3;8(6):e66220. doi: 10.1371/journal.pone.0066220. Print 2013. PLoS One. 2013. PMID: 23755301 Free PMC article. - Lessons from Retinoblastoma: Implications for Cancer, Development, Evolution, and Regenerative Medicine.
Dyer MA. Dyer MA. Trends Mol Med. 2016 Oct;22(10):863-876. doi: 10.1016/j.molmed.2016.07.010. Epub 2016 Aug 23. Trends Mol Med. 2016. PMID: 27567287 Free PMC article. Review. - Association of Hearing Impairment With Neurocognition in Survivors of Childhood Cancer.
Bass JK, Liu W, Banerjee P, Brinkman TM, Mulrooney DA, Gajjar A, Pappo AS, Merchant TE, Armstrong GT, Srivastava D, Robison LL, Hudson MM, Krull KR. Bass JK, et al. JAMA Oncol. 2020 Sep 1;6(9):1363-1371. doi: 10.1001/jamaoncol.2020.2822. JAMA Oncol. 2020. PMID: 32729886 Free PMC article. - Association of hearing loss with patient-reported functional outcomes in adult survivors of childhood cancer.
Bass JK, Wang F, Thaxton ME, Warren SE, Srivastava DK, Hudson MM, Ness KK, Brinkman TM. Bass JK, et al. J Natl Cancer Inst. 2024 Apr 5;116(4):596-605. doi: 10.1093/jnci/djad250. J Natl Cancer Inst. 2024. PMID: 38048603 Free PMC article. - Different infusion durations for preventing platinum-induced hearing loss in children with cancer.
van As JW, van den Berg H, van Dalen EC. van As JW, et al. Cochrane Database Syst Rev. 2018 Jul 5;7(7):CD010885. doi: 10.1002/14651858.CD010885.pub4. Cochrane Database Syst Rev. 2018. PMID: 29975402 Free PMC article. Updated. Review.
References
- Rodriguez-Galindo C, Wilson MW, Haik BG, et al. Treatment of intraocular retinoblastoma with vincristine and carboplatin. J Clin Oncol. 2003;21:2019–2025. - PubMed
- Jehanne M, Lumbroso-Le Rouic L, Savignoni A, et al. Analysis of ototoxicity in young children receiving carboplatin in the context of conservative management of unilateral or bilateral retinoblastoma. Pediatr Blood Cancer. 2009;52:637–643. - PubMed
- Lambert MP, Shields C, Meadows AT. A retrospective review of hearing in children with retinoblastoma treated with carboplatin-based chemotherapy. Pediatr Blood Cancer. 2008;50:223–226. - PubMed
- Smits C, Swen SJ, Theo Goverts S, et al. Assessment of hearing in very young children receiving carboplatin for retinoblastoma. Eur J Cancer. 2006;42:492–500. - PubMed
- Friedman DL, Himelstein B, Shields CL, et al. Chemoreduction and local ophthalmic therapy for intraocular retinoblastoma. J Clin Oncol. 2000;18:12–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources