Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3 - PubMed (original) (raw)
Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3
Barak Blum et al. Nat Biotechnol. 2012.
Abstract
Insulin-expressing cells that have been differentiated from human pluripotent stem cells in vitro lack the glucose responsiveness characteristic of mature beta cells. Beta-cell maturation in mice was studied to find genetic markers that enable screens for factors that induce bona fide beta cells in vitro. We find that functional beta-cell maturation is marked by an increase in the glucose threshold for insulin secretion and by expression of the gene urocortin 3.
Figures
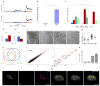
Figure 1. β-cell maturation is defined by a decrease in GSIS sensitivity to low glucose levels and by the expression of Ucn3
(A) Three independent sets of 50 islets each, from P1 or P15 mice, were sequentially perfused with basal (0.5mM, gray), low (2.8mM, blue) or high (16.7mM, red) glucose in a dynamic GSIS assay. Arrows indicate the time points at which solutions were changed. P1 islets display complete first and second phases of GSIS in response to low glucose, whereas P15 islets do not secrete insulin at this glucose concentration. (B) Triplicates of 10 islets from P1 to adult were assayed for GSIS using low glucose (2.8mM) and high glucose (16.7mM). Two age groups can be distinguished according to their stimulation index (fold change in GSIS). ***, P<5×10−5. (C) Three independent sets of ten islets each from P1, P9 or P21 were assayed for GSIS using low glucose (2.8mM, blue), high glucose (16.7mM, red), 20mM arginine (gray) or 30mM KCl (green). The difference in the amount of insulin secreted between mature and immature islets is specific to glucose. *, P<0.05; **, P<0.001; NS, not significant). (D) Blood glucose and (E) insulin levels in immature (P1, blue) and mature (P14, red) mouse pups. Insulin levels in the immature pups are higher than in the mature ones, although their blood glucose levels are lower. (F) Electron micrograph of insulin vesicles in β-cells at various ages. Scale bars = 2μm. (G) Quantification of the number of insulin vesicles vs. β-cell area of the data shown in F. (H) A scheme representing the microarray approach. Genes differentially expressed in both mature age groups compared to both immature age groups (I and II) are chosen as candidate markers. (I) Representative scattered plot from the microarray. Note high similarity (R2) in gene expression between the mature (P10) and immature (P1) samples. (J) The expression levels of most β-cell markers are unchanged during GSIS maturation. Scatter plots of global gene expression from microarrays on FACS-sorted immature (P1) and mature (P10) β-cells. Red lines mark a 2-fold difference in expression and, with the exception of MafB, gene expression is not significantly different between these stages. (K) The expression of Ucn3 mRNA at various ages as detected in the microarray. (L) Immunostaining of Ucn3 (green) and insulin (red) on pancreata from E18.5 and adult mice. Nuclei are stained with DAPI (blue). Scale bars = 50μm (M) Ucn3 is undetectable in E18.5 embryo. Ucn3 is detected at high levels and co-localizes with adult β-cells.
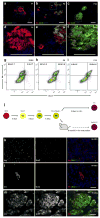
Figure 2. Ucn3 expression gradually increases during the course of mouse β-cell maturation in vivo and is expressed in HESC-derived β-like cells after differentiation and maturation in vivo, but not after differentiation in vitro.
(A–C) Immunostaining of Ucn3 (green) and insulin (red) on pancreata from P1, P6 and P22 mice. (D–F) Enlargement insets shown in A–C, respectively. Nuclei are stained with DAPI (blue). Scale bars = 50μm. (A, D) Ucn3 in not detected at P1 even in large islets. (B, E) At P6, some large islets express Ucn3, but small aggregates do not express the peptide (arrows). (D, F) At P22, Ucn3 is highly expressed in all islets. (G–I) Intra-cellular FACS analysis of insulin and Ucn3 at E18.5, P6 and P13. Numbers in upper quadrants represent the percentage of insulin only (left) or insulin and Ucn3 co-expressing cells (right) of all insulin-expressing cells (two upper quadrants), calculated as average±sem of three independent biological repeats (three separate litters) for each age group. (J) An outline of the experimental approach on HESCs differentiation. HESCs (ES, red) marked by Oct4 were differentiated in vitro into definitive endoderm (DE, yellow) marked by Sox17 and subsequently to pancreatic progenitors (PP, green), marked by the expression of Pdx1 and NKX6.1. The cells were transplanted into SCID-beige mice to complete maturation in vivo. (K, L) Immunostaining for Ucn3 (green) and insulin (red) on the in vitro differentiated cells shown at two magnifications (K, low magnification; L, high magnification). In vivo differentiated (transplanted) cells are shown in (M). Nuclei are stained with DAPI (blue). Scale bars = 50μm. Ucn3 is expressed in the in vivo matured cells, but not in in vitro differentiated insulin-positive β-like cells.
Similar articles
- Genetic deletion of Urocortin 3 does not prevent functional maturation of beta cells.
Huang JL, Lee S, Hoek P, van der Meulen T, Van R, Huising MO. Huang JL, et al. J Endocrinol. 2020 Jul;246(1):69-78. doi: 10.1530/JOE-19-0535. J Endocrinol. 2020. PMID: 32369775 Free PMC article. - Maturation of stem cell-derived beta-cells guided by the expression of urocortin 3.
van der Meulen T, Huising MO. van der Meulen T, et al. Rev Diabet Stud. 2014 Spring;11(1):115-32. doi: 10.1900/RDS.2014.11.115. Epub 2014 May 10. Rev Diabet Stud. 2014. PMID: 25148370 Free PMC article. Review. - ROCKII inhibition promotes the maturation of human pancreatic beta-like cells.
Ghazizadeh Z, Kao DI, Amin S, Cook B, Rao S, Zhou T, Zhang T, Xiang Z, Kenyon R, Kaymakcalan O, Liu C, Evans T, Chen S. Ghazizadeh Z, et al. Nat Commun. 2017 Aug 21;8(1):298. doi: 10.1038/s41467-017-00129-y. Nat Commun. 2017. PMID: 28824164 Free PMC article. - Urocortin 3 marks mature human primary and embryonic stem cell-derived pancreatic alpha and beta cells.
van der Meulen T, Xie R, Kelly OG, Vale WW, Sander M, Huising MO. van der Meulen T, et al. PLoS One. 2012;7(12):e52181. doi: 10.1371/journal.pone.0052181. Epub 2012 Dec 14. PLoS One. 2012. PMID: 23251699 Free PMC article. - Urocortin3: Local inducer of somatostatin release and bellwether of beta cell maturity.
Flisher MF, Shin D, Huising MO. Flisher MF, et al. Peptides. 2022 May;151:170748. doi: 10.1016/j.peptides.2022.170748. Epub 2022 Jan 19. Peptides. 2022. PMID: 35065098 Free PMC article. Review.
Cited by
- Inhibitory Effect of TCF7L2 on Pancreatic β-Cell Dedifferentiation via ERK/MAPK Signaling Pathway in Diabetes.
Wu HH, Ma QW, Liu YM, Wu X, Wen J. Wu HH, et al. Clin Med Insights Endocrinol Diabetes. 2024 Oct 26;17:11795514241295620. doi: 10.1177/11795514241295620. eCollection 2024. Clin Med Insights Endocrinol Diabetes. 2024. PMID: 39473826 Free PMC article. - Circadian Regulation of the Pancreatic Beta Cell.
Seshadri N, Doucette CA. Seshadri N, et al. Endocrinology. 2021 Sep 1;162(9):bqab089. doi: 10.1210/endocr/bqab089. Endocrinology. 2021. PMID: 33914056 Free PMC article. Review. - MAFA and T3 Drive Maturation of Both Fetal Human Islets and Insulin-Producing Cells Differentiated From hESC.
Aguayo-Mazzucato C, DiIenno A, Hollister-Lock J, Cahill C, Sharma A, Weir G, Colton C, Bonner-Weir S. Aguayo-Mazzucato C, et al. J Clin Endocrinol Metab. 2015 Oct;100(10):3651-9. doi: 10.1210/jc.2015-2632. Epub 2015 Jul 24. J Clin Endocrinol Metab. 2015. PMID: 26207953 Free PMC article. - Dynamic chromatin remodeling mediated by polycomb proteins orchestrates pancreatic differentiation of human embryonic stem cells.
Xie R, Everett LJ, Lim HW, Patel NA, Schug J, Kroon E, Kelly OG, Wang A, D'Amour KA, Robins AJ, Won KJ, Kaestner KH, Sander M. Xie R, et al. Cell Stem Cell. 2013 Feb 7;12(2):224-37. doi: 10.1016/j.stem.2012.11.023. Epub 2013 Jan 11. Cell Stem Cell. 2013. PMID: 23318056 Free PMC article. - ISR inhibition reverses pancreatic β-cell failure in Wolfram syndrome models.
Hu R, Chen X, Su Q, Wang Z, Wang X, Gong M, Xu M, Le R, Gao Y, Dai P, Zhang ZN, Shao L, Li W. Hu R, et al. Cell Death Differ. 2024 Mar;31(3):322-334. doi: 10.1038/s41418-024-01258-w. Epub 2024 Feb 6. Cell Death Differ. 2024. PMID: 38321214 Free PMC article.
References
- Kroon E, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. - PubMed
- Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn. 240:530–565. - PubMed
- Slack JM. Developmental biology of the pancreas. Development. 1995;121:1569–1580. - PubMed
- Rozzo A, Meneghel-Rozzo T, Delakorda SL, Yang SB, Rupnik M. Exocytosis of insulin: in vivo maturation of mouse endocrine pancreas. Ann N Y Acad Sci. 2009;1152:53–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases