5'-AMP Activated Protein Kinase is Involved in the Regulation of Myocardial β-Oxidative Capacity in Mice - PubMed (original) (raw)
doi: 10.3389/fphys.2012.00033. eCollection 2012.
Steen Larsen, Jonas Thue Treebak, Christina Neigaard Hansen, Martin Hey-Mogensen, Tobias Speerschneider, Thomas E Jensen, Jacob Jeppesen, Jørgen F P Wojtaszewski, Erik A Richter, Lars Køber, Flemming Dela
Affiliations
- PMID: 22371704
- PMCID: PMC3284200
- DOI: 10.3389/fphys.2012.00033
5'-AMP Activated Protein Kinase is Involved in the Regulation of Myocardial β-Oxidative Capacity in Mice
Nis Stride et al. Front Physiol. 2012.
Abstract
5'-adenosine monophosphate-activated protein kinase (AMPK) is considered central in regulation of energy status and substrate utilization within cells. In heart failure the energetic state is compromised and substrate metabolism is altered. We hypothesized that this could be linked to changes in AMPK activity and we therefore investigated mitochondrial oxidative phosphorylation capacity from the oxidation of long- and medium-chain fatty acids (LCFA and MCFA) in cardiomyocytes from young and old mice expressing a dominant negative AMPKα2 (AMPKα2-KD) construct and their wildtype (WT) littermates. We found a 35-45% (P < 0.05) lower mitochondrial capacity for oxidizing MCFA in AMPKα2-KD of both age-groups, compared to WT. This coincided with marked decreases in protein expression (19/29%, P < 0.05) and activity (14/21%, P < 0.05) of 3-hydroxyacyl-CoA-dehydrogenase (HAD), in young and old AMPKα2-KD mice, respectively, compared to WT. Maximal LCFA oxidation capacity was similar in AMPKα2-KD and WT mice independently of age implying that LCFA-transport into the mitochondria was unaffected by loss of AMPK activity or progressing age. Expression of regulatory proteins of glycolysis and glycogen breakdown showed equivocal effects of age and genotype. These results illustrate that AMPK is necessary for normal mitochondrial function in the heart and that decreased AMPK activity may lead to an altered energetic state as a consequence of reduced capacity to oxidize MCFA. We did not identify any clear aging effects on mitochondrial function.
Keywords: AMPK; metabolic remodeling; mitochondria; oxidative phosphorylation.
Figures
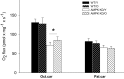
Figure 1
Mitochondrial respiratory capacity in heart muscle samples from wild type mice (WT) and dominant negative AMPKα2 (AMPK KD) mice. Young (Y: 19–22 weeks) and old (O: 76–91 weeks) mice were studied in both groups. State 3 respiration was measured with two different substrate protocols. Malate, ADP, and octanoylcarnitine (Oct.car) or malate, ADP, and palmitoylcarnitine (Pal.car). Values are mean ± SE. *Different from WT/Y (P < 0.05).
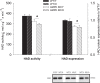
Figure 2
Citrate synthase (CS) enzyme activity (left _Y_-axis) and mitochondrial DNA content (right _Y_-axis) in heart muscle samples from wild type mice (WT) and dominant negative AMPKα2 (AMPK KD) mice. Young (Y: 19–22 weeks) and old (O: 76–91 weeks) mice were studied in both groups. *Different from age matched wild type (P < 0.05).
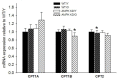
Figure 3
Carnitine palmitoyl transferase isoforms 1A (CPT1A) and 1B (CPT1B) as well as carnitine palmitoyl transferase 2 (CPT2) in heart muscle samples from wild type mice (WT) and dominant negative AMPKα2 (AMPK KD) mice. Young (Y: 19–22 weeks) and old (O: 76–91 weeks) mice were studied in both groups. *Different from WT/Y (P < 0.05).
Figure 4
3-Hydroxyacyl-CoA-dehydrogenase (HAD) enzyme activity (left _Y_-axis) and protein expression relative to WT/Y (right _Y_-axis). *Different from WT/Y (P < 0.05).
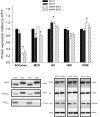
Figure 5
Protein content (Western blot) of pS212 acetyl-CoA-carboxylase (ACCphos) and malonyl-CoA-decarboxylase (MCD), glycogen-phosphorylase (GP), hexokinase II (HKII), and Pyruvatkinase (PKM) in heart muscle samples from wild type mice (WT) and dominant negative AMPKα2 (AMPK KD) mice. Young (Y: 19–22 weeks) and old (O: 76–91 weeks) mice were studied in both groups. Representative blots are shown in the lower panels, which also include blots of AMPK and AMPKphos. *Different from WT/Y (P < 0.05).
Similar articles
- Crucial role for LKB1 to AMPKalpha2 axis in the regulation of CD36-mediated long-chain fatty acid uptake into cardiomyocytes.
Habets DD, Coumans WA, El Hasnaoui M, Zarrinpashneh E, Bertrand L, Viollet B, Kiens B, Jensen TE, Richter EA, Bonen A, Glatz JF, Luiken JJ. Habets DD, et al. Biochim Biophys Acta. 2009 Mar;1791(3):212-9. doi: 10.1016/j.bbalip.2008.12.009. Epub 2008 Dec 30. Biochim Biophys Acta. 2009. PMID: 19159696 - Role of the α2 subunit of AMP-activated protein kinase and its nuclear localization in mitochondria and energy metabolism-related gene expressions in C2C12 cells.
Okamoto S, Asgar NF, Yokota S, Saito K, Minokoshi Y. Okamoto S, et al. Metabolism. 2019 Jan;90:52-68. doi: 10.1016/j.metabol.2018.10.003. Epub 2018 Oct 23. Metabolism. 2019. PMID: 30359677 - Two weeks of metformin treatment enhances mitochondrial respiration in skeletal muscle of AMPK kinase dead but not wild type mice.
Kristensen JM, Larsen S, Helge JW, Dela F, Wojtaszewski JF. Kristensen JM, et al. PLoS One. 2013;8(1):e53533. doi: 10.1371/journal.pone.0053533. Epub 2013 Jan 14. PLoS One. 2013. PMID: 23341947 Free PMC article. - AMP-activated protein kinase regulation of fatty acid oxidation in the ischaemic heart.
Hopkins TA, Dyck JR, Lopaschuk GD. Hopkins TA, et al. Biochem Soc Trans. 2003 Feb;31(Pt 1):207-12. doi: 10.1042/bst0310207. Biochem Soc Trans. 2003. PMID: 12546686 Review. - Regulation of energy metabolism by long-chain fatty acids.
Nakamura MT, Yudell BE, Loor JJ. Nakamura MT, et al. Prog Lipid Res. 2014 Jan;53:124-44. doi: 10.1016/j.plipres.2013.12.001. Epub 2013 Dec 18. Prog Lipid Res. 2014. PMID: 24362249 Review.
Cited by
- AMPKα2 Overexpression Reduces Cardiomyocyte Ischemia-Reperfusion Injury Through Normalization of Mitochondrial Dynamics.
Deng Y, Chen S, Zhang M, Li C, He J, Tan Y. Deng Y, et al. Front Cell Dev Biol. 2020 Aug 27;8:833. doi: 10.3389/fcell.2020.00833. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32984328 Free PMC article. - Tissue-specific and substrate-specific mitochondrial bioenergetics in feline cardiac and skeletal muscles.
Christiansen LB, Dela F, Koch J, Yokota T. Christiansen LB, et al. J Vet Med Sci. 2015 Jun;77(6):669-75. doi: 10.1292/jvms.14-0573. Epub 2015 Mar 11. J Vet Med Sci. 2015. PMID: 25716052 Free PMC article. - Caprylic Acid Inhibits High Mobility Group Box-1-Induced Mitochondrial Damage in Myocardial Tubes.
Nukaga S, Fujiwara-Tani R, Nishida R, Miyagawa Y, Goto K, Kawahara I, Nakashima C, Fujii K, Ogata R, Ohmori H, Kuniyasu H. Nukaga S, et al. Int J Mol Sci. 2024 Jul 24;25(15):8081. doi: 10.3390/ijms25158081. Int J Mol Sci. 2024. PMID: 39125651 Free PMC article. - AMPK maintains energy homeostasis and survival in cancer cells via regulating p38/PGC-1α-mediated mitochondrial biogenesis.
Chaube B, Malvi P, Singh SV, Mohammad N, Viollet B, Bhat MK. Chaube B, et al. Cell Death Discov. 2015 Dec 21;1:15063. doi: 10.1038/cddiscovery.2015.63. eCollection 2015. Cell Death Discov. 2015. PMID: 27551487 Free PMC article. - Molecular and metabolic mechanisms of cardiac dysfunction in diabetes.
Mandavia CH, Aroor AR, Demarco VG, Sowers JR. Mandavia CH, et al. Life Sci. 2013 Mar 28;92(11):601-8. doi: 10.1016/j.lfs.2012.10.028. Epub 2012 Nov 9. Life Sci. 2013. PMID: 23147391 Free PMC article. Review.
References
- Arad M., Benson D. W., Perez-Atayde A. R., McKenna W. J., Sparks E. A., Kanter R. J., McGarry K., Seidman J. G., Seidman C. E. (2002). Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy 1. J. Clin. Invest. 109, 357–36210.1172/JCI200214571 - DOI - PMC - PubMed
- Chandler M. P., Kerner J., Huang H., Vazquez E., Reszko A., Martini W. Z., Hoppel C. L., Imai M., Rastogi S., Sabbah H. N., Stanley W. C. (2004). Moderate severity heart failure does not involve a downregulation of myocardial fatty acid oxidation. Am. J. Physiol. Heart Circ. Physiol. 287, H1538–H154310.1152/ajpheart.00281.2004 - DOI - PubMed
LinkOut - more resources
Full Text Sources