GluA2-lacking AMPA receptors in hippocampal CA1 cell synapses: evidence from gene-targeted mice - PubMed (original) (raw)
GluA2-lacking AMPA receptors in hippocampal CA1 cell synapses: evidence from gene-targeted mice
Andrei Rozov et al. Front Mol Neurosci. 2012.
Abstract
The GluA2 subunit in heteromeric alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor channels restricts Ca(2+) permeability and block by polyamines, rendering linear the current-voltage relationship of these glutamate-gated cation channels. Although GluA2-lacking synaptic AMPA receptors occur in GABA-ergic inhibitory neurons, hippocampal CA1 pyramidal cell synapses are widely held to feature only GluA2 containing AMPA receptors. A controversy has arisen from reports of GluA2-lacking AMPA receptors at hippocampal CA3-to-CA1 cell synapses and a study contesting these findings. Here we sought independent evidence for the presence of GluA2-lacking AMPA receptors in CA1 pyramidal cell synapses by probing the sensitivity of their gated cation channels in wild-type (WT) mice and gene-targeted mouse mutants to philanthotoxin, a specific blocker of GluA2-lacking AMPA receptors. The mutants either lacked GluA2 for maximal philanthotoxin sensitivity, or, for minimal sensitivity, expressed GluA1 solely in a Q/R site-edited version or not at all. Our comparative electrophysiological analyses provide incontrovertible evidence for the presence in wild-type CA1 pyramidal cell synapses of GluA2-less AMPA receptor channels. This article is part of a Special Issue entitled "Calcium permeable AMPARs in synaptic plasticity and disease."
Keywords: AMPA receptors; hippocampal CA1 cell synapses.
Figures
Figure 1
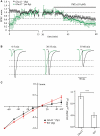
Maximal and polyamine-attenuated levels of PhTx-433 blockade in CA1 pyramidal cells of _GluA2_−/− mice. (A) The time course of EPSC amplitude changes during washout of polyamine and subsequent PhTx-433 (10 μM) application, when CA1 pyramidal neurons were dialyzed with polyamine-free intracellular solution (green circles). Also shown is the effect of exogenously added spermine on EPSC amplitudes and the masking effect of spermine on PhTx-433 suppression (black circles). (B) Representative averaged traces at the indicated times of recording in polyamine-free conditions are shown in green, in polyamine-containing condition in black. (C) Effect of intracellular polyamines on AMPAR-mediated synaptic currents in _GluA2_−/− and WT mice. AMPAR-mediated synaptic I–V curves measured with polyamine-containing intracellular solution in _GluA2_−/− and WT mice (left panel). Rectification properties of synaptic AMPAR channels in _GluA2_−/− and WT mice (right panel). RIs were calculated as the ratio of EPSC amplitudes measured at 30 and −70 mV (EPSC−70/EPSC30).
Figure 2
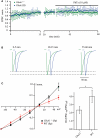
Synaptic AMPAR channels in CA1 pyramidal cells of _GluA1_−/− and GluA1(R) mouse mutants are polyamine and PhTx-433 insensitive. (A) The time course of EPSC amplitude changes recorded in CA1 pyramidal neurons of _GluA1_−/− (green circles) and GluA1(R) (blue circles) mutant mice during washout of polyamine and subsequent PhTx-433 (10 μM) application. (B) Representative averaged traces at the indicated times of recording from _GluA1_−/− mice shown in green, and for GluA1(R) mutants in blue. (C) Effect of intracellular polyamines on rectification properties of AMPAR-mediated synaptic currents in _GluA1_−/− and WT animals. Synaptic AMPAR-mediated I–V curves measured with polyamine-containing intracellular solution in _GluA1_−/− and WT mice (left panel). Rectification indices of synaptic AMPAR channels in _GluA2_−/− and WT mice (right panel).
Figure 3
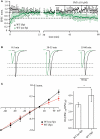
PhTx-433 sensitive AMPA channels in WT CA1 pyramidal cells. (A) Scatter plot shows EPSC amplitude dynamics during prolonged whole-cell dialysis and following PhTx-433 (10 μM) application, when CA1 pyramidal neurons were patched with either polyamine-free (green circles) or polyamine-containing (black circles) intracellular solution. (B) Representative averaged traces at the indicated times of recording in polyamine-free (green) or polyamine-containing (black) solution. (C) Dependence of AMPAR synaptic channel rectification properties in WT CA1 pyramidal cells on intracellular polyamine content. Synaptic AMPAR-mediated I–V curves measured with polyamine-containing and -free intracellular solutions (left panel). Dependence of rectification indices of synaptic AMPAR channels on the presence of polyamines in WT mice (right panel).
Figure 4
Washout of polyamines increases EPSC amplitudes. (A) Polyamines enter and block GluA2-lacking AMPARs in the closed state. In _GluA2_−/− mice almost all channels are blocked prior to glutamate release (left panel). During unitary synaptic events the channel conductance is strongly attenuated, resulting in reduced amplitudes of the net response (middle panel). In contrast, washout of polyamines results in an increased AMPAR conductance, leading to enhanced EPSC amplitudes (right panel). (B) When GluA2-lacking and GluA2-containing AMPARs are co-expressed at the same synapse, the effect of polyamine washout will depend on the ratio between the two channel types. Representative traces show enhancement of AMPAR-mediated EPSCs during polyamine washout (WT in red, _GluA2_−/− in black). (C) The plot summarizes data on the initial amplitude enhancement observed in WT, _GluA2_−/− and _GluA1_−/− mice. The dashed line shows the exponential fits of the time dependent amplitude augmentation during polyamine washout (WT in red, _GluA2_−/− in black and _GluA1_−/− in blue). Since the vast majority of CA1 cell AMPA channels in _GluA1_−/− mice should be polyamine insensitive, the slight EPSC enhancement represents another intrinsic property of Schaffer collateral/commisural to CA1 pyramidal cells synapses (e.g., frequency facilitation). Thus, by subtracting the _GluA1_−/− data from those obtained from WT or _GluA2_−/− mice, we can calculate the putative effect of endogenous polyamine washout on EPSC amplitudes in WT (red line) and _GluA2_−/− (black line) mice.
Figure A1
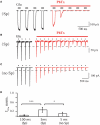
(A) Effect of PhTx application on GluA1-medeated currents in outside-out patches excised from HEK 293 cells. Duration of each glutamate application was 100 ms; holding potential was 70 mV; PA-containing pipette solution was used. Currents evoked before PhTx-433 application are shown in black and those recorded in the presence of toxin in red. For analysis current amplitudes at steady-state level of suppression were normalized to the control amplitude measured prior PhTx-433 application (0.03 ± 0.01; n = 5). (B) The same as in (A), but duration of each glutamate application was 5 ms. Averaged level of suppression was 0.28 ± 0.11 (n = 5). (C) The same as in (B), but PA-free pipette solution was used. Averaged level of suppression was 0.11 ± 0.07 (n = 5). (D) Bar histogram compares the effect of long (100 ms) and sort (5 ms) duration of glutamate application and effect of the presence of polyamines on the level of PhTx suppression.
Similar articles
- Evidence of calcium-permeable AMPA receptors in dendritic spines of CA1 pyramidal neurons.
Mattison HA, Bagal AA, Mohammadi M, Pulimood NS, Reich CG, Alger BE, Kao JP, Thompson SM. Mattison HA, et al. J Neurophysiol. 2014 Jul 15;112(2):263-75. doi: 10.1152/jn.00578.2013. Epub 2014 Apr 23. J Neurophysiol. 2014. PMID: 24760782 Free PMC article. - A new mouse line with reduced GluA2 Q/R site RNA editing exhibits loss of dendritic spines, hippocampal CA1-neuron loss, learning and memory impairments and NMDA receptor-independent seizure vulnerability.
Konen LM, Wright AL, Royle GA, Morris GP, Lau BK, Seow PW, Zinn R, Milham LT, Vaughan CW, Vissel B. Konen LM, et al. Mol Brain. 2020 Feb 27;13(1):27. doi: 10.1186/s13041-020-0545-1. Mol Brain. 2020. PMID: 32102661 Free PMC article. - Stargazin (TARP gamma-2) is required for compartment-specific AMPA receptor trafficking and synaptic plasticity in cerebellar stellate cells.
Jackson AC, Nicoll RA. Jackson AC, et al. J Neurosci. 2011 Mar 16;31(11):3939-52. doi: 10.1523/JNEUROSCI.5134-10.2011. J Neurosci. 2011. PMID: 21411637 Free PMC article. - GluA2-lacking, calcium-permeable AMPA receptors--inducers of plasticity?
Man HY. Man HY. Curr Opin Neurobiol. 2011 Apr;21(2):291-8. doi: 10.1016/j.conb.2011.01.001. Epub 2011 Feb 2. Curr Opin Neurobiol. 2011. PMID: 21295464 Free PMC article. Review. - Redefining the classification of AMPA-selective ionotropic glutamate receptors.
Bowie D. Bowie D. J Physiol. 2012 Jan 1;590(1):49-61. doi: 10.1113/jphysiol.2011.221689. Epub 2011 Nov 21. J Physiol. 2012. PMID: 22106175 Free PMC article. Review.
Cited by
- Persistent synaptic scaling independent of AMPA receptor subunit composition.
Altimimi HF, Stellwagen D. Altimimi HF, et al. J Neurosci. 2013 Jul 17;33(29):11763-7. doi: 10.1523/JNEUROSCI.1102-13.2013. J Neurosci. 2013. PMID: 23864664 Free PMC article. - Acquisition of contextual discrimination involves the appearance of a RAS-GRF1/p38 mitogen-activated protein (MAP) kinase-mediated signaling pathway that promotes long term potentiation (LTP).
Jin SX, Arai J, Tian X, Kumar-Singh R, Feig LA. Jin SX, et al. J Biol Chem. 2013 Jul 26;288(30):21703-13. doi: 10.1074/jbc.M113.471904. Epub 2013 Jun 13. J Biol Chem. 2013. PMID: 23766509 Free PMC article. - AMPA-Type Glutamate Receptor Conductance Changes and Plasticity: Still a Lot of Noise.
Benke T, Traynelis SF. Benke T, et al. Neurochem Res. 2019 Mar;44(3):539-548. doi: 10.1007/s11064-018-2491-1. Epub 2018 Feb 23. Neurochem Res. 2019. PMID: 29476449 Free PMC article. Review. - Evidence of calcium-permeable AMPA receptors in dendritic spines of CA1 pyramidal neurons.
Mattison HA, Bagal AA, Mohammadi M, Pulimood NS, Reich CG, Alger BE, Kao JP, Thompson SM. Mattison HA, et al. J Neurophysiol. 2014 Jul 15;112(2):263-75. doi: 10.1152/jn.00578.2013. Epub 2014 Apr 23. J Neurophysiol. 2014. PMID: 24760782 Free PMC article. - Intracellular Ca²⁺ and not the extracellular matrix determines surface dynamics of AMPA-type glutamate receptors on aspiny neurons.
Klueva J, Gundelfinger ED, Frischknecht RR, Heine M. Klueva J, et al. Philos Trans R Soc Lond B Biol Sci. 2014 Oct 19;369(1654):20130605. doi: 10.1098/rstb.2013.0605. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 25225098 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous