Central B-cell tolerance: where selection begins - PubMed (original) (raw)
Central B-cell tolerance: where selection begins
Roberta Pelanda et al. Cold Spring Harb Perspect Biol. 2012.
Abstract
The development of an adaptive immune system based on the random generation of antigen receptors requires a stringent selection process that sifts through receptor specificities to remove those reacting with self-antigens. In the B-cell lineage, this selection process is first applied to IgM(+) immature B cells. By using increasingly sophisticated mouse models, investigators have identified the central tolerance mechanisms that negatively select autoreactive immature B cells and prevent inclusion of their antigen receptors into the peripheral B-cell pool. Additional studies have uncovered mechanisms that promote the differentiation of nonautoreactive immature B cells and their positive selection into the peripheral B-cell population. These mechanisms of central selection are fundamental to the generation of a naïve B-cell repertoire that is largely devoid of self-reactivity while capable of reacting with any foreign insult.
Figures
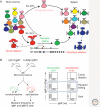
Figure 1.
Schematic representation of B-cell development and Ig loci in mice. Large pro-B cells initiate Ig gene rearrangement at the IgH locus. Expression of a H chain following a productive _V_H_D_H_J_H recombination event promotes the differentiation of large pre-B cells in which the expression of pre-BCR (H chain pairing with surrogate light chains) results in the clonal expansion of H chain-positive pre-B cells and the development of small pre-B cells. Expression of conventional L chains following productive rearrangements at the IgL chain loci in small pre-B cells promotes the development of a diverse population of IgM+ immature B cells, which then differentiate into IgM+IgD+ transitional B cells. The scheme of mouse Ig H, κ, and λ loci (not to scale) indicate the presence of V (white rectangles), D (black vertical lines), J (brown vertical lines; a dashed line indicates a nonfunctional element), and C (black rectangles; a gray rectangle indicates a nonfunctional element) gene segments. The scheme does not represent the number of _V_H, _D_H, and _V_κ gene segments in the actual Ig loci.
Figure 2.
Receptor editing in central B-cell selection. (A) Schematic representation of central B-cell tolerance. Immature B cells reacting with low to high avidity self-antigens undergo receptor editing, here represented by a secondary rearrangement at the Igκ allele. Immature B cells reacting with low avidity self-antigens can alternatively further differentiate and migrate into the spleen as anergic or ignorant B cells. Clonal deletion that occurs at a frequency that is presently unknown, but that is likely very low, is represented as a by-product of cells undergoing failed receptor editing. B cells encountering self-antigen in the periphery are represented undergoing peripheral deletion. (B) Experimental setup that tested the relative contribution of receptor editing and clonal deletion to central tolerance of developing 3-83Ig+ B cells (Halverson et al. 2004). Bone marrow cells from wild-type IgMb congenic and 3-83Igi IgMa congenic mice were mixed at equal proportion and injected into lethally irradiated recipient mice of H-2d and H-2b genetic backgrounds. The frequency of IgMa and IgMb B cells in the total B-cell population was measured in mixed bone marrow chimeras of the two experimental groups. The scheme on the right represents the expected outcomes of this analysis if all anti-Kb 3-83Ig+ B cells had undergone clonal deletion (top panel), receptor editing (middle panel), or a combination of either tolerance mechanism (bottom panel) in mice expressing the self-antigen (H-2b), and relative to nonautoreactive mice (H-2d). The blue rectangle indicates the experimental findings.
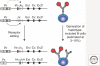
Figure 3.
Receptor editing generates a small population of haplotype-included B cells. During receptor editing, a potential rearrangement at the second Igκ allele (intact arrow), or at the IgH or Igλ alleles, results in the generation of cells coexpressing two or more types of H and L chains. Some of these haplotype-included B cells are selected into the peripheral B-cell population expressing both autoreactive and nonautoreactive antibodies. Note that if receptor editing occurs on the original rearranged Igκ allele (dotted arrow), the previously used _V_-J sequence would be deleted.
Figure 4.
Positive selection of nonautoreactive immature B cells into the peripheral B-cell compartment requires a threshold level of tonic BCR signaling. Differentiation of nonautoreactive immature B cells into transitional and mature B cells and entry into the peripheral B-cell population depends on a certain threshold of BCR expression and tonic BCR signaling, which is translated by the Ras-Mek-Erk signaling pathway. BAFFR expression correlates with BCR surface expression and tonic signaling, and BAFFR signaling contributes to the differentiation of immature into transitional B cells. The scheme is based on data from Rowland et al. 2010a,.
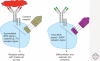
Figure 5.
Autoreactive and nonautoreactive immature B cells undergo different fates during bone marrow selection. An autoreactive immature B-cell (on the left) experiences antigen-mediated BCR signaling in addition to the absence of tonic BCR signaling, and these events promote ongoing Ig gene rearrangements (receptor editing). Cytokines, such as IL-7, may be able to sustain limited cell survival during the editing process. A nonautoreactive immature B cell (on the right) experiences tonic BCR signaling in addition to BAFFR signaling, and these events promote further cell differentiation and selection into the peripheral B-cell compartment.
Similar articles
- Receptor editing can lead to allelic inclusion and development of B cells that retain antibodies reacting with high avidity autoantigens.
Liu S, Velez MG, Humann J, Rowland S, Conrad FJ, Halverson R, Torres RM, Pelanda R. Liu S, et al. J Immunol. 2005 Oct 15;175(8):5067-76. doi: 10.4049/jimmunol.175.8.5067. J Immunol. 2005. PMID: 16210610 - Antigen and cytokine receptor signals guide the development of the naïve mature B cell repertoire.
Rowland SL, Tuttle K, Torres RM, Pelanda R. Rowland SL, et al. Immunol Res. 2013 Mar;55(1-3):231-40. doi: 10.1007/s12026-012-8366-7. Immunol Res. 2013. PMID: 22941591 Free PMC article. Review. - Activation of Ras overcomes B-cell tolerance to promote differentiation of autoreactive B cells and production of autoantibodies.
Teodorovic LS, Babolin C, Rowland SL, Greaves SA, Baldwin DP, Torres RM, Pelanda R. Teodorovic LS, et al. Proc Natl Acad Sci U S A. 2014 Jul 8;111(27):E2797-806. doi: 10.1073/pnas.1402159111. Epub 2014 Jun 23. Proc Natl Acad Sci U S A. 2014. PMID: 24958853 Free PMC article. - B-cell intrinsic and extrinsic signals that regulate central tolerance of mouse and human B cells.
Pelanda R, Greaves SA, Alves da Costa T, Cedrone LM, Campbell ML, Torres RM. Pelanda R, et al. Immunol Rev. 2022 May;307(1):12-26. doi: 10.1111/imr.13062. Epub 2022 Jan 8. Immunol Rev. 2022. PMID: 34997597 Free PMC article. Review.
Cited by
- V(D)J Recombination Exploits DNA Damage Responses to Promote Immunity.
Arya R, Bassing CH. Arya R, et al. Trends Genet. 2017 Jul;33(7):479-489. doi: 10.1016/j.tig.2017.04.006. Epub 2017 May 19. Trends Genet. 2017. PMID: 28532625 Free PMC article. Review. - Minding the gap: The impact of B-cell tolerance on the microbial antibody repertoire.
Finney J, Watanabe A, Kelsoe G, Kuraoka M. Finney J, et al. Immunol Rev. 2019 Nov;292(1):24-36. doi: 10.1111/imr.12805. Epub 2019 Sep 27. Immunol Rev. 2019. PMID: 31559648 Free PMC article. Review. - Understanding and measuring human B-cell tolerance and its breakdown in autoimmune disease.
Cashman KS, Jenks SA, Woodruff MC, Tomar D, Tipton CM, Scharer CD, Eun-Hyung Lee F, Boss JM, Sanz I. Cashman KS, et al. Immunol Rev. 2019 Nov;292(1):76-89. doi: 10.1111/imr.12820. Epub 2019 Nov 22. Immunol Rev. 2019. PMID: 31755562 Free PMC article. Review. - Autoimmune lymphoproliferative immunodeficiencies (ALPID) in childhood: breakdown of immune homeostasis and immune dysregulation.
Toskov V, Ehl S. Toskov V, et al. Mol Cell Pediatr. 2023 Sep 13;10(1):11. doi: 10.1186/s40348-023-00167-1. Mol Cell Pediatr. 2023. PMID: 37702894 Free PMC article. Review. - B-cell tolerance and autoimmunity.
Tsubata T. Tsubata T. F1000Res. 2017 Mar 29;6:391. doi: 10.12688/f1000research.10583.1. eCollection 2017. F1000Res. 2017. PMID: 28408984 Free PMC article. Review.
References
- Ait-Azzouzene D, Verkoczy L, Duong B, Skog P, Gavin AL, Nemazee D 2006. Split tolerance in peripheral B cell subsets in mice expressing a low level of Igκ-reactive ligand. J Immunol 176: 939–948 - PubMed
- Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR 2001. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol 167: 6834–6840 - PubMed
- Arakawa H, Shimizu T, Takeda S 1996. Re-evaluation of the probabilities for productive arrangements on the κ and λ loci. Int Immunol 8: 91–99 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI052310/AI/NIAID NIH HHS/United States
- AI052157/AI/NIAID NIH HHS/United States
- AA022295/AA/NIAAA NIH HHS/United States
- AI078468/AI/NIAID NIH HHS/United States
- P01 AI022295/AI/NIAID NIH HHS/United States
- R56 AI052310/AI/NIAID NIH HHS/United States
- R21 AI078468/AI/NIAID NIH HHS/United States
- R01 AI052310/AI/NIAID NIH HHS/United States
- R01 AI052157/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources