Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage - PubMed (original) (raw)
Comparative Study
Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage
Jie Liu et al. J Neurosci. 2012.
Abstract
Blood-brain barrier (BBB) disruption occurs early enough to be within the thrombolytic time window, and this early ischemic BBB damage is closely associated with hemorrhagic transformation and thus emerging as a promising target for reducing the hemorrhagic complications of thrombolytic stroke therapy. However, the mechanisms underlying early ischemic BBB damage remain poorly understood. Here, we investigated the early molecular events of ischemic BBB damage using in vitro oxygen-glucose deprivation (OGD) and in vivo rat middle cerebral artery occlusion (MCAO) models. Exposure of bEND3 monolayer to OGD for 2 h significantly increased its permeability to FITC-labeled dextran and promoted the secretion of metalloproteinase-2 and -9 (MMP-2/9) and cytosolic translocation of caveolin-1 (Cav-1). This same OGD treatment also led to rapid degradation of tight junction protein occludin and dissociation of claudin-5 from the cytoskeleton, which contributed to OGD-induced endothelial barrier disruption. Using selective MMP-2/9 inhibitor SB-3CT (2-[[(4-phenoxyphenyl)sulfonyl]methyl]-thiirane) or their neutralizing antibodies or Cav-1 siRNA, we found that MMP-2 was the major enzyme mediating OGD-induced occludin degradation, while Cav-1 was responsible for claudin-5 redistribution. The interaction between Cav-1 and claudin-5 was further confirmed by coimmunoprecipitation. Consistent with these in vitro findings, we observed fluorescence tracer extravasation, increased gelatinolytic activity, and elevated interstitial MMP-2 levels in ischemic subcortical tissue after 2 h MCAO. Moreover, occludin protein loss and claudin-5 redistribution were detected in ischemic cerebromicrovessels. These data indicate that cerebral ischemia initiates two rapid parallel processes, MMP-2-mediated occludin degradation and Cav-1-mediated claudin-5 redistribution, to cause BBB disruption at early stroke stages relevant to acute thrombolysis.
Figures
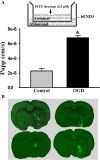
Figure 1.
Ischemia causes rapid disruption of the BBB in vitro and in vivo. A, Top panel, Schematic representation of the in vitro BBB model (bEND3 monolayer grown on an insert) with FITC-dextran loaded in the luminal compartment. Bottom panel, The endothelial monolayer permeability was assessed by calculating the transfer rate of FITC-dextran from luminal compartment to abluminal compartment and was expressed as apparent permeability coefficient (Papp) (in centimeters per second). Exposure of bEND3 monolayer to OGD for 2 h significantly increased its permeability to FITC-dextran. *p < 0.05 versus control cultures, Student's t test; n = 6. B, Cerebral ischemia rapidly induced BBB disruption in the ischemic brain of all tested rats (n = 6). Representative fluorescence micrographs of brain cryosections from four different rats revealed that 2 h MCAO induced FITC-albumin extravasation (bright green fluorescence) in the subcortical regions in the ischemic hemisphere. No FITC-albumin leakage was observed in other brain regions. Error bars indicate SEM.
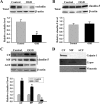
Figure 2.
OGD induces rapid degradation of occludin and redistribution of claudin-5 in bEND3 cells. After exposing bEND3 cells to OGD for 2 h, total cell lysates and extracts of subcellular fractions were prepared and subjected to Western blot analysis for tight junction proteins occludin and claudin-5. As a loading control, the blots were stripped and reprobed with β-actin antibody. A, B, Two hour OGD induced a significant reduction in total occludin protein level but did not change the total protein level of claudin-5. C, OGD induced redistribution of claudin-5 between subcellular compartments, as reflected by a remarkable reduction in claudin-5 levels in the ACF and a significant increase of its levels in the CF and MF. The blots for CF were stripped and reprobed with β-actin antibody. D, The specificity of each fraction was confirmed with anti-calpain (CF), anti-Cypor (MF), and anti-vimentin (ACF) antibodies. *p < 0.05 versus control, Student's t test; n = 4. Error bars indicate SEM.
Figure 3.
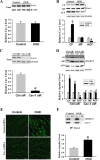
OGD-induced occludin degradation is MMP-2/9 dependent. A, OGD rapidly elevated MMP-2/9 levels in conditioned media. After exposure of bEND3 cells to OGD for 2 h, a significant increase in MMP-2/9 levels was detected in the conditioned medium on gelatin zymograms when compared with the control cultures (Ctrl). Active MMP-2 (the bottom band), but no active MMP-9, was seen on zymogram gels. MMP-2 or -9 levels were quantified by measuring the sum intensity of their latent and active bands. *p < 0.05 versus Ctrl, Student's t test; n = 5. Std, Standard human MMP-2/9. B, Selective MMP-2/9 inhibitor SB-3CT completely inhibited OGD-induced occludin degradation. bEND3 cells were treated with SB-3CT (10 μ
m
) 2 h before and during 2 h OGD. Occludin protein in total cellular extracts was detected with Western blot. β-Actin served as a loading control. *p < 0.05 versus vehicle (DMSO) plus Ctrl, #p < 0.05 versus vehicle plus OGD, ANOVA; n = 5. C, MMP-2 neutralizing antibody (M2) completely inhibited OGD-induced occludin degradation, while no significant effects were observed for IgG or MMP-9 neutralizing antibody (M9). bEND3 cells were treated with 20 μg/ml control mouse IgG, MMP-2 or MMP-9 neutralizing antibodies, or both (M2,9) during 2 h OGD. Occludin protein in total cellular extracts was detected with Western blot. β-Actin served as a loading control. *p < 0.05 versus control (Ctrl), #p < 0.05 versus OGD alone (−) or OGD plus IgG, ANOVA; n = 4. D, Representative confocal micrographs showed MMP-dependent degradation of occludin. Control bEND3 cells revealed a circumcellular immunostaining of occludin, which was significantly reduced after exposing cells to OGD for 2 h. SB-3CT treatment completely inhibited occludin reduction in OGD-treated cells. Experiments were repeated three times with similar results. Scale bar, 20 μm. E, Inhibition of MMP-2/9 with SB-3CT had no effect on OGD-induced claudin-5 redistribution. Claudin-5 proteins in subcellular fractions were detected with Western blot. *p < 0.05 versus vehicle plus Ctrl, ANOVA; n = 5. Error bars indicate SEM.
Figure 4.
OGD elevates extracellular MMP-2/9 levels through promoting their secretion from the preexisting intracellular pool in bEND3 cells. A, Gelatin zymography analysis showed that 2 h OGD markedly increased MMP-2/9 levels in the CM, which was accompanied by a significant reduction in their levels in whole CEs. *p < 0.05 versus control, Student's t test; n = 5. Std, Human MMP-2/9 standards. B, Real-time RT-PCR analysis showed that 2 h OGD did not change MMP-2/9 mRNA expression in bEND3 cells. n = 5. C, D, Inhibition of mRNA synthesis with Act D or inhibition of protein synthesis with CHX did not significantly affect OGD-induced MMP-2/9 secretion in bEND3 cells. Cells were treated with Act D (2 μg/ml) or CHX (100 μg/ml) or vehicle (DMSO) 1 h before and during 2 h OGD treatment. *p < 0.05 versus control (Ctrl), ANOVA; n = 5. E, Two hour OGD also stimulates MMP-2/9 secretion from astrocytic cell line C8-D1A (left panel) and neuronal cell line SH-SY5Y (right panel), reflected by increased MMP-2/9 in CM and concurrent reduction of their levels in CEs. Experiments are repeated four times with similar results. Error bars indicate SEM.
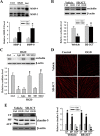
Figure 5.
Cav-1 mediates OGD-induced claudin-5 dissociation from the cytoskeleton in endothelial cells. A, B, Western blot analysis showed that exposing bEND3 cells to OGD for 2 h had no effects on the total protein levels of Cav-1, but triggered its redistribution among subcellular fractions, as reflected by increased Cav-1 level in the CF and decreased level in the ACF. *p < 0.05 versus control, Student's t test; n = 5. C, Cav-1 siRNA effectively knocked down Cav-1 protein expression in bEND3 cells. Western blot analysis showed that incubation cells with Cav-1 siRNA (Cav-1 siR) for 48 h significantly (∼90%) reduced Cav-1 protein levels. *p < 0.05 versus control siRNA (Ctrl siR), Student's t test; n = 5. D, Knockdown of Cav-1 with siRNA prevented OGD-induced claudin-5 redistribution in bEND3 cells. Following OGD treatment, claudin-5 levels in CF, MF were increased, while its level in ACF was markedly reduced, which was inhibited by Cav-1 siRNA. *p < 0.05 versus control (Ctrl); #p < 0.05 versus Ctrl siR plus OGD, ANOVA; n = 5. E, Representative confocal microscope images revealed a circumcellular immunostaining of claudin-5 in control bEND3 cells. Two hour OGD treatment did not change the immunostaining intensity of claudin-5, but significantly disturbed its normal distribution pattern. Knockdown of Cav-1 with siRNA inhibited claudin-5 redistribution induced by OGD. Experiments were repeated three times with similar results. Scale bar, 20 μm. F, Representative immunoblot of coimmunoprecipitation of Cav-1 and claudin-5 from whole-cell lysates of control cultures or OGD-treated cells with anti-Cav-1 antibody or normal IgG (top panel). Bottom panel, OGD enhanced interaction of Cav-1 with claudin-5. *p < 0.05 versus control, Student's t test; n = 4. Error bars indicate SEM.
Figure 6.
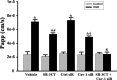
Inhibition of MMP-2/9 with SB-3CT or knockdown of Cav-1 with siRNA reduces OGD-induced BBB disruption in vitro. The permeability of FITC-dextran across bEND3 monolayers was significantly increased after 2 h exposure to OGD, which was partially inhibited by pretreating cells with MMP-2/9 inhibitor SB3-CT or Cav-1 siRNA. Combination of SB-3CT and Cav-1 siRNA completely preserved the endothelial barrier integrity of OGD-treated endothelial monolayer. The endothelial monolayer permeability was assessed by calculating the transfer rate of FITC-dextran from luminal compartment to abluminal compartment, and was expressed as apparent permeability coefficient (Papp) (in centimeters per second). *p < 0.05 versus control; #p < 0.05 versus vehicle-OGD cultures; φ_p_ < 0.05 versus control siRNA (Ctrl siR) plus OGD; &p < 0.05 versus Cav-1 siRNA (Cav-1 siR) plus OGD or SB-3CT plus OGD, ANOVA; n = 6. Error bars indicate SEM.
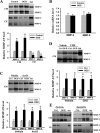
Figure 7.
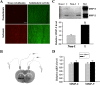
Cerebral ischemia rapidly increases extracellular MMP-2/9 levels and their activities in ischemic striatum. After 2 h MCAO, the gelatinolytic activity of MMP-2/9 and their extracellular levels were analyzed by in situ zymography and microdialysis sampling/gel zymography, respectively. A, In situ zymography was performed on cryosections obtained from brain tissue injected with Texas Red-albumin. Increased gelatinolytic activity of MMP-2/9 was found in the ischemic striatal tissue (bright green fluorescence), where Texas Red-albumin leakage concurrently occurred. No tracer leakage and weak gelatinolytic activity were seen in the corresponding nonischemic striatal tissue. Scale bar, 50 μm. Experiments were repeated four times with similar results. B, Schematic diagram of in vivo microdialysis sampling, by which MMP-2/9 in the interstitial space of the nonischemic (Non-I) and ischemic (I) striatum were collected during 2 h MCAO. C, Gel gelatin zymography analysis of collected dialysates showed that MMP-2/9, particularly MMP-2, were significantly increased in the interstitial space of the ischemic striatum. *p < 0.05 versus Non-I, Student's t test; n = 4. D, MMP-2/9 mRNA expression was not changed in the ischemic striatal tissue after 2 h MCAO (n = 6). Total RNA was extracted from nonischemic and ischemic striatal tissues and mRNA expression was analyzed by real-time RT-PCR. Error bars indicate SEM.
Figure 8.
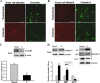
Cerebral ischemia induces rapid occludin degradation and claudin-5 redistribution in vivo. After 2 h MCAO, the protein levels of occludin and claudin-5 in cerebral microvessels were analyzed by IHC or Western blot. A, B, Immunostaining for occludin and claudin-5 was performed on cryosections obtained from brain tissue injected with Texas Red-albumin. Immunostaining (green) for occludin and claudin-5 were clearly seen on the microvessels of the nonischemic tissue, where no Texas Red-albumin leakage was observed. In the ischemic hemisphere, tracer leakage was accompanied by reduced occludin staining on the microvessels, while no appreciable changes were observed for claudin-5 staining. Scale bar, 25 μm. Experiments were repeated four times with similar results. C, D, Cerebral microvessels were isolated from nonischemic (Non-I) and ischemic (I) hemispheric tissue after 2 h MCAO. Total microvascular extracts and subcellular fractions were prepared for analyzing occludin and claudin-5 protein levels with Western blot. As a loading control, the blots were stripped and reprobed with β-actin antibody. MCAO induced a significant reduction in occludin levels in total microvascular extracts (C). *p < 0.05 versus Non-I, Student's t test; n = 6. MCAO did not change total claudin-5 levels in cerebral microvascular extracts (top left panel) but led to a remarkable reduction in claudin-5 level in the ACF and its significant increases in the CF and MF (top right and bottom panels). *p < 0.05 versus Non-I, Student's t test; n = 4. Error bars indicate SEM.
Similar articles
- Notoginsenoside R1 intervenes degradation and redistribution of tight junctions to ameliorate blood-brain barrier permeability by Caveolin-1/MMP2/9 pathway after acute ischemic stroke.
Liu B, Li Y, Han Y, Wang S, Yang H, Zhao Y, Li P, Wang Y. Liu B, et al. Phytomedicine. 2021 Sep;90:153660. doi: 10.1016/j.phymed.2021.153660. Epub 2021 Jul 25. Phytomedicine. 2021. PMID: 34344565 - Comparative study of extracellular vesicles derived from mesenchymal stem cells and brain endothelial cells attenuating blood-brain barrier permeability via regulating Caveolin-1-dependent ZO-1 and Claudin-5 endocytosis in acute ischemic stroke.
Li Y, Liu B, Zhao T, Quan X, Han Y, Cheng Y, Chen Y, Shen X, Zheng Y, Zhao Y. Li Y, et al. J Nanobiotechnology. 2023 Feb 28;21(1):70. doi: 10.1186/s12951-023-01828-z. J Nanobiotechnology. 2023. PMID: 36855156 Free PMC article. - Autophagy-mediated occludin degradation contributes to blood-brain barrier disruption during ischemia in bEnd.3 brain endothelial cells and rat ischemic stroke models.
Kim KA, Kim D, Kim JH, Shin YJ, Kim ES, Akram M, Kim EH, Majid A, Baek SH, Bae ON. Kim KA, et al. Fluids Barriers CNS. 2020 Mar 14;17(1):21. doi: 10.1186/s12987-020-00182-8. Fluids Barriers CNS. 2020. PMID: 32169114 Free PMC article. - Extracellular Vesicles Maintain Blood-Brain Barrier Integrity by the Suppression of Caveolin-1/CD147/VEGFR2/MMP Pathway After Ischemic Stroke.
Li Y, Chen J, Quan X, Chen Y, Han Y, Chen J, Yang L, Xu Y, Shen X, Wang R, Zhao Y. Li Y, et al. Int J Nanomedicine. 2024 Feb 13;19:1451-1467. doi: 10.2147/IJN.S444009. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38371456 Free PMC article. - Blood-brain barrier in acute liver failure.
Nguyen JH. Nguyen JH. Neurochem Int. 2012 Jun;60(7):676-83. doi: 10.1016/j.neuint.2011.10.012. Epub 2011 Nov 13. Neurochem Int. 2012. PMID: 22100566 Free PMC article. Review.
Cited by
- Noise alters guinea pig's blood-labyrinth barrier ultrastructure and permeability along with a decrease of cochlear Claudin-5 and Occludin.
Wu YX, Zhu GX, Liu XQ, Sun F, Zhou K, Wang S, Wang CM, Jia JW, Song JT, Lu LJ. Wu YX, et al. BMC Neurosci. 2014 Dec 24;15:136. doi: 10.1186/s12868-014-0136-0. BMC Neurosci. 2014. PMID: 25539640 Free PMC article. - Cerebral Microvascular Endothelial Cell Apoptosis after Ischemia: Role of Enolase-Phosphatase 1 Activation and Aci-Reductone Dioxygenase 1 Translocation.
Zhang Y, Wang T, Yang K, Xu J, Ren L, Li W, Liu W. Zhang Y, et al. Front Mol Neurosci. 2016 Aug 31;9:79. doi: 10.3389/fnmol.2016.00079. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27630541 Free PMC article. - Blood-brain barrier dysfunction in disorders of the developing brain.
Moretti R, Pansiot J, Bettati D, Strazielle N, Ghersi-Egea JF, Damante G, Fleiss B, Titomanlio L, Gressens P. Moretti R, et al. Front Neurosci. 2015 Feb 17;9:40. doi: 10.3389/fnins.2015.00040. eCollection 2015. Front Neurosci. 2015. PMID: 25741233 Free PMC article. Review. - Caveolin-1 in the anterior cingulate cortex modulates chronic neuropathic pain via regulation of NMDA receptor 2B subunit.
Yang JX, Hua L, Li YQ, Jiang YY, Han D, Liu H, Tang QQ, Yang XN, Yin C, Hao LY, Yu L, Wu P, Shao CJ, Ding HL, Zhang YM, Cao JL. Yang JX, et al. J Neurosci. 2015 Jan 7;35(1):36-52. doi: 10.1523/JNEUROSCI.1161-14.2015. J Neurosci. 2015. PMID: 25568101 Free PMC article. - ADAM12 and ADAM17 are essential molecules for hypoxia-induced impairment of neural vascular barrier function.
Cui D, Arima M, Takubo K, Kimura T, Horiuchi K, Minagawa T, Matsuda S, Ikeda E. Cui D, et al. Sci Rep. 2015 Aug 5;5:12796. doi: 10.1038/srep12796. Sci Rep. 2015. PMID: 26242473 Free PMC article.
References
- Alberts MJ. tPA in acute ischemic stroke: United States experience and issues for the future. Neurology. 1998;51:S53–S55. - PubMed
- András IE, Pu H, Deli MA, Nath A, Hennig B, Toborek M. HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J Neurosci Res. 2003;74:255–265. - PubMed
- Aviv RI, d'Esterre CD, Murphy BD, Hopyan JJ, Buck B, Mallia G, Li V, Zhang L, Symons SP, Lee TY. Hemorrhagic transformation of ischemic stroke: prediction with CT perfusion. Radiology. 2009;250:867–877. - PubMed
- Bang OY, Buck BH, Saver JL, Alger JR, Yoon SR, Starkman S, Ovbiagele B, Kim D, Ali LK, Sanossian N, Jahan R, Duckwiler GR, Viñuela F, Salamon N, Villablanca JP, Liebeskind DS. Prediction of hemorrhagic transformation after recanalization therapy using T2*-permeability magnetic resonance imaging. Ann Neurol. 2007;62:170–176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous