A mouse model for MeCP2 duplication syndrome: MeCP2 overexpression impairs learning and memory and synaptic transmission - PubMed (original) (raw)
Comparative Study
A mouse model for MeCP2 duplication syndrome: MeCP2 overexpression impairs learning and memory and synaptic transmission
Elisa S Na et al. J Neurosci. 2012.
Abstract
Rett syndrome and MECP2 duplication syndrome are neurodevelopmental disorders that arise from loss-of-function and gain-of-function alterations in methyl-CpG binding protein 2 (MeCP2) expression, respectively. Although there have been studies examining MeCP2 loss of function in animal models, there is limited information on MeCP2 overexpression in animal models. Here, we characterize a mouse line with MeCP2 overexpression restricted to neurons (Tau-Mecp2). This MeCP2 overexpression line shows motor coordination deficits, heightened anxiety, and impairments in learning and memory that are accompanied by deficits in long-term potentiation and short-term synaptic plasticity. Whole-cell voltage-clamp recordings of cultured hippocampal neurons from Tau-Mecp2 mice reveal augmented frequency of miniature EPSCs with no change in miniature IPSCs, indicating that overexpression of MeCP2 selectively impacts excitatory synapse function. Moreover, we show that alterations in transcriptional repression mechanisms underlie the synaptic phenotypes in hippocampal neurons from the Tau-Mecp2 mice. These results demonstrate that the Tau-Mecp2 mouse line recapitulates many key phenotypes of MECP2 duplication syndrome and support the use of these mice to further study this devastating disorder.
Figures
Figure 1.
Increased expression of MeCP2 in Tau–Mecp2 mice compared with WT control littermates. A, B, Immunofluorescence images of the CA1 and dentate gyrus (DG) areas of the hippocampus. Left column shows MeCP2 protein in WT control littermates. Right column shows MeCP2 in Tau–Mecp2 mice. Top row is at a 10× magnification, and the bottom row is at a 40× magnification. C, Western blot data demonstrating an increase in MeCP2 protein expression in Tau–Mecp2 mice in different areas of the brain. FC, Frontal cortex; CPu, caudate–putamen; HC, hippocampus; CBL, cerebellum. WT mice, n = 4; Tau–Mecp2 mice, n = 4. *p < 0.05.
Figure 2.
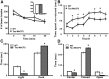
Behavioral phenotypes observed in mice that overexpress Mecp2. A, Locomotor activity is not significantly different between WT and Tau–Mecp2 mice. B, Tau–Mecp2 mice spend significantly less time on the rotarod on trials 2–4, 6, and 7 compared with WT mice, demonstrating motor coordination deficits. Significant main effects of time (F(7,315) = 19.5, p < 0.05) and genotype (F(7,315) = 8.2, p < 0.05). Post hoc Fisher's LSD tests revealed significant differences between Tau–Mecp2 and WT mice at trials 2–4, 6, and 7. C, DL test demonstrating an increased anxiety-like phenotype in mice that overexpress Mecp2 compared with WT mice (t(21) = 4.99, p < 0.05). Tau–Mecp2 mice spend significantly less time in the light side than WT mice. D, EPM data showing increased anxiety-like behavior in Tau–Mecp2 mice, which spend less time in the open arms and more time in the closed arms compared with WT controls (t(21) = 3.30, p < 0.05). WT mice, n = 11;Tau–Mecp2 mice, n = 12. *p < 0.05 between Tau–Mecp2 and WT mice.
Figure 3.
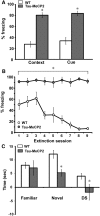
Learning and memory deficits in Tau–Mecp2 mice. A, Tau–Mecp2 mice (n = 12) show increased freezing responses in both context and cue fear conditioning compared with WT (n = 11) littermates (t(20) = 7.4, t(20) = 6.3, p < 0.05, respectively). B, Tau–Mecp2 mice do not learn to extinguish freezing behavior to repeated presentations of cue after nine extinction sessions, whereas WT control littermates extinguish freezing responses by the fourth extinction session, indicating learning and memory deficits in Tau–Mecp2 mice (WT, n = 7; Tau–Mecp2, n = 6). A repeated-measures ANOVA revealed a significant interaction effect between trials and genotype (F(8,125) = 4.8, p < 0.05), and a Fisher's LSD analysis revealed significant differences between all extinction trials. C, Mice that overexpress Mecp2 are not able to distinguish between a novel object and a familiar one. Tau–Mecp2 mice spend less time exploring the novel object than WT mice (t(22) = 3.4, p < 0.05) and also spend less time with the novel object versus the familiar object as demonstrated by their significant negative difference score (DS) (t(22) = 2.1, p < 0.05). There is no difference, however, in the amount of time spent with the familiar object between Tau–Mecp2 and WT mice. *p < 0.05 between WT mice and Tau–Mecp2 mice.
Figure 4.
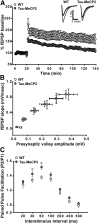
LTP induced by HFS is impaired in Tau–Mecp2 hippocampal slices. A, Tau–Mecp2 mice show attenuated LTP responses induced by HFS compared with WT controls (WT, n = 16; Tau–Mecp2, n = 11). A significant time × genotype interaction effect was seen (F(271,7343) = 3.0, p < 0.05). Post hoc comparisons using Fisher's LSD revealed differences at almost all time points after HFS except for 15 time points of the 78 time points analyzed. Representative traces are shown in the inset (black, before stimulation; red, after LTP stimulation). B, There were no significant differences in the slope of the I/O relationship between Tau–Mecp2 and WT mice (WT, n = 8; Tau–Mecp2, n = 10). C, Tau–Mecp2 hippocampal slices show impairments in PPF. PPF was significantly augmented by MeCP2 overexpression, with Tau–Mecp2 mice showing increased PPF at interstimulus intervals 30 ms (t(16) = 2.2, p < 0.05) and 50 ms (t(16) = 3.1, p < 0.05) compared with WT controls, indicating a decreased probability of neurotransmitter release (WT, n = 8; Tau–Mecp2, n = 10). *p < 0.05 between WT mice and Tau–Mecp2 mice.
Figure 5.
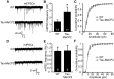
Increased excitatory neurotransmission in mice that overexpress Mecp2. A, Representative traces of mEPSC frequency in WT and Tau–Mecp2 hippocampal neuronal cultures. B, mEPSC frequency was significantly increased in hippocampal Tau–Mecp2 cultures compared with WT cultures (*p < 0.05). C, There were no significant differences in the cumulative distribution of mEPSC amplitudes. D, Representative traces of mIPSC frequency in WT and Tau–Mecp2 hippocampal neurons. E, There were no significant differences in mIPSC frequency. F, Cumulative distribution of mIPSC amplitudes was not significantly altered in Tau–Mecp2 hippocampal neurons. The number of cells recorded in mEPSC and mIPSC experiments are shown in the respective bar graph.
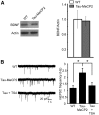
Figure 6.
Inhibition of transcriptional repressor processes rescue the synaptic deficits in hippocampal neurons from Tau–Mecp2 mice. A, BDNF protein expression was quantified relative to actin in Tau–Mecp2 and WT mice. There were no significant differences in relative BDNF protein expression in Tau–Mecp2 compared with WT mice (n = 4 for each group). B, Left, Representative traces of mEPSC frequency in WT and Tau–Mecp2 hippocampal neuronal cultures treated with TSA. B, The significant increase in mEPSC frequency seen in Tau–Mecp2 hippocampal neurons is reduced back to WT levels with 24 h TSA treatment (WT, n = 9; Tau–Mecp2, n = 8; Tau + TSA, n = 8; *p < 0.05).
Comment in
- Insights into the cellular and molecular contributions of MeCP2 overexpression to disease pathophysiology.
Taylor MM, Doshi S. Taylor MM, et al. J Neurosci. 2012 Jul 11;32(28):9451-3. doi: 10.1523/JNEUROSCI.2043-12.2012. J Neurosci. 2012. PMID: 22787029 Free PMC article. No abstract available.
Similar articles
- Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome.
Moretti P, Levenson JM, Battaglia F, Atkinson R, Teague R, Antalffy B, Armstrong D, Arancio O, Sweatt JD, Zoghbi HY. Moretti P, et al. J Neurosci. 2006 Jan 4;26(1):319-27. doi: 10.1523/JNEUROSCI.2623-05.2006. J Neurosci. 2006. PMID: 16399702 Free PMC article. - The impact of MeCP2 loss- or gain-of-function on synaptic plasticity.
Na ES, Nelson ED, Kavalali ET, Monteggia LM. Na ES, et al. Neuropsychopharmacology. 2013 Jan;38(1):212-9. doi: 10.1038/npp.2012.116. Epub 2012 Jul 11. Neuropsychopharmacology. 2013. PMID: 22781840 Free PMC article. Review. - Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome.
Asaka Y, Jugloff DG, Zhang L, Eubanks JH, Fitzsimonds RM. Asaka Y, et al. Neurobiol Dis. 2006 Jan;21(1):217-27. doi: 10.1016/j.nbd.2005.07.005. Epub 2005 Aug 8. Neurobiol Dis. 2006. PMID: 16087343 - Synaptic maturation at cortical projections to the lateral amygdala in a mouse model of Rett syndrome.
Gambino F, Khelfaoui M, Poulain B, Bienvenu T, Chelly J, Humeau Y. Gambino F, et al. PLoS One. 2010 Jul 2;5(7):e11399. doi: 10.1371/journal.pone.0011399. PLoS One. 2010. PMID: 20625482 Free PMC article. - The role of MeCP2 in CNS development and function.
Na ES, Monteggia LM. Na ES, et al. Horm Behav. 2011 Mar;59(3):364-8. doi: 10.1016/j.yhbeh.2010.05.014. Epub 2010 May 31. Horm Behav. 2011. PMID: 20515694 Free PMC article. Review.
Cited by
- Neuronal morphology in MeCP2 mouse models is intrinsically variable and depends on age, cell type, and Mecp2 mutation.
Wang IT, Reyes AR, Zhou Z. Wang IT, et al. Neurobiol Dis. 2013 Oct;58:3-12. doi: 10.1016/j.nbd.2013.04.020. Epub 2013 May 6. Neurobiol Dis. 2013. PMID: 23659895 Free PMC article. - miR-132 couples the circadian clock to daily rhythms of neuronal plasticity and cognition.
Aten S, Hansen KF, Price KH, Wheaton K, Kalidindi A, Garcia A, Alzate-Correa D, Hoyt KR, Obrietan K. Aten S, et al. Learn Mem. 2018 Apr 16;25(5):214-229. doi: 10.1101/lm.047191.117. Print 2018 May. Learn Mem. 2018. PMID: 29661834 Free PMC article. - Ultrafast simulation of large-scale neocortical microcircuitry with biophysically realistic neurons.
Oláh VJ, Pedersen NP, Rowan MJM. Oláh VJ, et al. Elife. 2022 Nov 7;11:e79535. doi: 10.7554/eLife.79535. Elife. 2022. PMID: 36341568 Free PMC article. - Cytogenetic and molecular characterization of a recombinant X chromosome in a family with a severe neurologic phenotype and macular degeneration.
Magini P, Poscente M, Ferrari S, Vargiolu M, Bacchelli E, Graziano C, Wischmeijer A, Turchetti D, Malaspina E, Marchiani V, Cordelli DM, Franzoni E, Romeo G, Seri M. Magini P, et al. Mol Cytogenet. 2015 Aug 1;8:58. doi: 10.1186/s13039-015-0164-1. eCollection 2015. Mol Cytogenet. 2015. PMID: 26236399 Free PMC article. - Synaptopathology Involved in Autism Spectrum Disorder.
Guang S, Pang N, Deng X, Yang L, He F, Wu L, Chen C, Yin F, Peng J. Guang S, et al. Front Cell Neurosci. 2018 Dec 21;12:470. doi: 10.3389/fncel.2018.00470. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30627085 Free PMC article. Review.
References
- Asaka Y, Jugloff DG, Zhang L, Eubanks JH, Fitzsimonds RM. Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol Dis. 2006;21:217–227. - PubMed
- Barbosa AC, Kim MS, Ertunc M, Adachi M, Nelson ED, McAnally J, Richardson JA, Kavalali ET, Monteggia LM, Bassel-Duby R, Olson EN. MEF2C, a transcription factor that facilitates learning and memory by negative regulation of synapse numbers and function. Proc Natl Acad Sci U S A. 2008;105:9391–9396. - PMC - PubMed
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study “recognition memory.”. Nat Protoc. 2006;1:1306–1311. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous