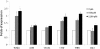Effect of intracellular lipid accumulation in a new model of non-alcoholic fatty liver disease - PubMed (original) (raw)
Effect of intracellular lipid accumulation in a new model of non-alcoholic fatty liver disease
Norberto C Chavez-Tapia et al. BMC Gastroenterol. 2012.
Abstract
Background: In vitro exposure of liver cells to high concentrations of free fatty acids (FFA) results in fat overload which promotes inflammatory and fibrogenic response similar to those observed in patients with Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH). Since the mechanisms of this event have not been fully characterized, we aimed to analyze the fibrogenic stimuli in a new in vitro model of NASH.
Methods: HuH7 cells were cultured for 24 h in an enriched medium containing bovine serum albumin and increasing concentrations of palmitic and oleic acid at a molar ratio of 1:2 (palmitic and oleic acid, respectively). Cytotoxic effect, apoptosis, oxidative stress, and production of inflammatory and fibrogenic cytokines were measured.
Results: FFA induces a significant increment in the intracellular content of lipid droplets. The gene expression of interleukin-6, interleukin-8 and tumor necrosis factor alpha was significantly increased. The protein level of interleukin-8 was also increased. Intracellular lipid accumulation was associated to a significant up-regulation in the gene expression of transforming growth factor beta 1, alpha 2 macroglobulin, vascular endothelial growth factor A, connective tissue growth factor, insulin-like growth factor 2, thrombospondin 1. Flow cytometry analysis demonstrated a significant increment of early apoptosis and production of reactive oxygen species.
Conclusions: The exposure of hepatocytes to fatty acids elicits inflammation, increase of oxidative stress, apoptosis and production of fibrogenic cytokines. These data support a primary role of FFA in the pathogenesis of NAFLD and NASH.
Figures
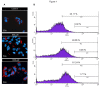
Figure 1
Dose dependent intracellular fat accumulation. Cell exposure to 600 and 1200 μM FFA for 24 h. Dose dependent intracellular fat accumulation evidenced by Nile Red staining assessed by fluorescence microscopy (A) and flow cytometry measured in 1 × 104 cells (B), M1 represents the percentage of events above the maximum value of fluorescence and M2 represents the percentage of events above the median value of fluorescence.
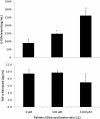
Figure 2
Inflammatory cytokines mRNA expression. Cells were cultured and treated with 600 and 1200 μM FFA for 24 h. mRNA expression of IL-6, IL-8 and TNF-alpha in HuH7 cells vs. control. * P < 0.05 versus control (0 μM).
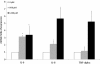
Figure 3
Inflammatory cytokines protein production. Inflammatory cytokines present in the supernatant of HuH7 cells after 24 h of the treatment was significantly increased only for IL-8 at both experimental doses, TNF-alpha levels were unchanged, and the levels of IL-6 were lower than the assay sensitivity. * P < 0.05 versus control (0 μM).
Figure 4
Fibrogenic cytokines mRNA expression. Fibrogenic cytokines mRNA expression in HuH7 cells after 24 h exposure at 600 and 1200 μM FFA. * P < 0.05 versus control (0 μM).
Figure 5
Apoptosis induction secondary to fatty acids toxicity. FFA treatment increase apoptosis in hepatic cells (HuH7). There is a reduction in the live cells, and in consequence an increase number of cells in early-, late-apoptosis and necrosis with the FFA treatment. The results are presented as percentage compared to control and represent the mean ± SD, of at least three independent experiments (A). Representative dotplot graph of control and 1200 μM FFA treated cells stained with Annexin V and PI (B).* P < 0.05 versus control (0 μM).
Similar articles
- A human hepatocellular in vitro model to investigate steatosis.
Gómez-Lechón MJ, Donato MT, Martínez-Romero A, Jiménez N, Castell JV, O'Connor JE. Gómez-Lechón MJ, et al. Chem Biol Interact. 2007 Jan 30;165(2):106-16. doi: 10.1016/j.cbi.2006.11.004. Epub 2006 Nov 23. Chem Biol Interact. 2007. PMID: 17188672 - The role of hepassocin in the development of non-alcoholic fatty liver disease.
Wu HT, Lu FH, Ou HY, Su YC, Hung HC, Wu JS, Yang YC, Wu CL, Chang CJ. Wu HT, et al. J Hepatol. 2013 Nov;59(5):1065-72. doi: 10.1016/j.jhep.2013.06.004. Epub 2013 Jun 18. J Hepatol. 2013. PMID: 23792031 - Cellular glutathione in fatty liver in vitro models.
Garcia MC, Amankwa-Sakyi M, Flynn TJ. Garcia MC, et al. Toxicol In Vitro. 2011 Oct;25(7):1501-6. doi: 10.1016/j.tiv.2011.05.011. Epub 2011 May 17. Toxicol In Vitro. 2011. PMID: 21620948 - Lipidomic biomarkers and mechanisms of lipotoxicity in non-alcoholic fatty liver disease.
Svegliati-Baroni G, Pierantonelli I, Torquato P, Marinelli R, Ferreri C, Chatgilialoglu C, Bartolini D, Galli F. Svegliati-Baroni G, et al. Free Radic Biol Med. 2019 Nov 20;144:293-309. doi: 10.1016/j.freeradbiomed.2019.05.029. Epub 2019 May 29. Free Radic Biol Med. 2019. PMID: 31152791 Review. - Non-Alcoholic Fatty Liver Disease.
Engin A. Engin A. Adv Exp Med Biol. 2017;960:443-467. doi: 10.1007/978-3-319-48382-5_19. Adv Exp Med Biol. 2017. PMID: 28585211 Review.
Cited by
- Role of thrombospondin 1 in liver diseases.
Li Y, Turpin CP, Wang S. Li Y, et al. Hepatol Res. 2017 Feb;47(2):186-193. doi: 10.1111/hepr.12787. Epub 2016 Aug 30. Hepatol Res. 2017. PMID: 27492250 Free PMC article. Review. - Glial cell line-derived neurotrophic factor-induced mice liver defatting: A novel strategy to enable transplantation of steatotic livers.
Taba Taba Vakili S, Kailar R, Rahman K, Nezami BG, Mwangi SM, Anania FA, Srinivasan S. Taba Taba Vakili S, et al. Liver Transpl. 2016 Apr;22(4):459-67. doi: 10.1002/lt.24385. Liver Transpl. 2016. PMID: 26714616 Free PMC article. - Paclitaxel Ameliorates Palmitate-Induced Injury in Mouse Podocytes.
Son SS, Kang JS, Lee EY. Son SS, et al. Med Sci Monit Basic Res. 2020 Dec 16;26:e928265. doi: 10.12659/MSMBR.928265. Med Sci Monit Basic Res. 2020. PMID: 33323915 Free PMC article. - Regulation of lipid droplet (LD) formation in hepatocytes via regulation of SREBP1c by non-coding RNAs.
El Sobky SA, Aboud NK, El Assaly NM, Fawzy IO, El-Ekiaby N, Abdelaziz AI. El Sobky SA, et al. Front Med (Lausanne). 2022 Sep 20;9:903856. doi: 10.3389/fmed.2022.903856. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36203751 Free PMC article. - Suppression of NASH-Related HCC by Farnesyltransferase Inhibitor through Inhibition of Inflammation and Hypoxia-Inducible Factor-1α Expression.
Yamada K, Tanaka T, Kai K, Matsufuji S, Ito K, Kitajima Y, Manabe T, Noshiro H. Yamada K, et al. Int J Mol Sci. 2023 Jul 17;24(14):11546. doi: 10.3390/ijms241411546. Int J Mol Sci. 2023. PMID: 37511305 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources