B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive immunity - PubMed (original) (raw)
B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive immunity
E Ashley Moseman et al. Immunity. 2012.
Abstract
Neutralizing antibodies have been thought to be required for protection against acutely cytopathic viruses, such as the neurotropic vesicular stomatitis virus (VSV). Utilizing mice that possess B cells but lack antibodies, we show here that survival upon subcutaneous (s.c.) VSV challenge was independent of neutralizing antibody production or cell-mediated adaptive immunity. However, B cells were absolutely required to provide lymphotoxin (LT) α1β2, which maintained a protective subcapsular sinus (SCS) macrophage phenotype within virus draining lymph nodes (LNs). Macrophages within the SCS of B cell-deficient LNs, or of mice that lack LTα1β2 selectively in B cells, displayed an aberrant phenotype, failed to replicate VSV, and therefore did not produce type I interferons, which were required to prevent fatal VSV invasion of intranodal nerves. Thus, although B cells are essential for survival during VSV infection, their contribution involves the provision of innate differentiation and maintenance signals to macrophages, rather than adaptive immune mechanisms.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
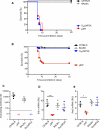
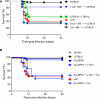
Figure 1. Antibodies, but Not B Cells, Are Dispensable for Protection against Subcutaneous VSV Infection
(A) Kaplan-Meier survival curves of C57BL/6 (n = 9), BALB/c (n = 10), μMT (C57BL/6 background; n = 5), and DHLMP2A (BALB/c background; n = 6) mice infected with 106 pfu of VSV-IND i.v. DHLMP2A versus μMT: p = 0.51, Log-rank (Mantel-Cox) test. (B) Kaplan-Meier survival curves of C57BL/6 (n = 93), BALB/c (n = 30), μMT (n = 50), and DHLMP2A (n = 36) mice infected with 104 pfu of VSV-IND i.fp. DHLMP2A versus μMT: p < 0.0001, Log-rank (Mantel-Cox) test. (C) VSV neutralizing antibody titers in the sera of the mice described in (B), 15 days after infection. Graph is representative of two experiments (n = 4 per experiment); ND = not detected. (D and E) Ifnb1 (D) and Ifna2 (E) mRNA levels in total RNA isolated from LNs of the animals described in (B) and sacrificed 8 hr p.i. Analysis was performed by quantitative RT-PCR and results are expressed as relative units (RU) after normalization to the mRNA content of the housekeeping gene GAPDH. Graphs are representative of two experiments (n = 3 per experiment). **p < 0.001, *p < 0.05.
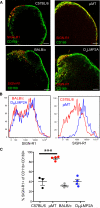
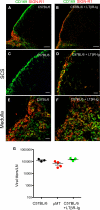
Figure 2. Surface Phenotype of SCS Macrophages in WT, μMT, and DHLMP2A Mice
(A) Representative confocal micrographs of frozen popliteal LN sections stained with MAbs against SIGN-R1 (red), and CD169 (green). Scale bars represent 100 μm. (B) FACS histograms of SIGN-R1 expression on CD11b+CD169+ LN cells of the indicated mouse strains. Histograms are representative of three experiments (n = 3–4 mice/experiment). (C) Percentage of SIGN-R1+ cells within the CD11b+CD169+ compartment in LNs of the indicated mouse strains. ***p < 0.001.
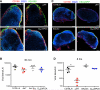
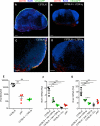
Figure 3. B Cells Are Required for VSV Replication in LN Macrophages
(A) Representative confocal micrographs of popliteal LN sections 30 min after footpad infection with Alexa Fluor 488-labeled VSV (VSV488, green). Sections were stained with MAbs against CD169 (red) and B220 (blue). Scale bars represent 100 μm. (B) Viral titers in popliteal LNs of mice infected 30 min earlier with VSV-IND into the footpad. Results are representative of two experiments (n = 4 per experiment). (C) Representative confocal micrographs of popliteal LN sections 8 hr after footpad infection with VSV-eGFP (green). Sections were stained as in (A). Scale bars represent 150 μm. (D) Viral titers in popliteal LNs 8 hr after footpad infection with VSV-IND. Results are representative of three experiments (n = 3–4 mice/experiment).
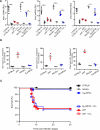
Figure 4. LN Macrophage Depletion Renders Antibody-Deficient Mice Susceptible to Fatal VSV Neuroinvasion
(A) Ifnb1 (left), Ifna2 (middle), and Ifna4 (right) mRNA levels in total RNA isolated from LNs of the indicated mouse strains, with or without clodronate liposomes (CLL) treatment, 8 hr after footpad infection with VSV-IND. Analysis was performed as indicated in Figures 1D and 1E. Graphs are representative of two experiments (n = 3–4 per experiment). ***p < 0.001, *p < 0.05. (B) The same set of data as in (A) are expressed as: (mRNA levels in CLL-treated LNs)/(mRNA levels in control LN) × 100. (C) Kaplan-Meier survival curves of C57BL/6 (n = 93), BALB/c (n = 30), and DHLMP2A with (n = 12) or without (n = 36) CLL treatment, and μMT with (n = 15) or without (n = 50) CLL treatment prior to footpad infection with VSV-IND. DHLMP2A versus DHLMP2A + CLL: p < 0.0001; μMT versus μMT + CLL: not significant; μMT versus DHLMP2A + CLL: not significant; Log-rank (Mantel-Cox) test.
Figure 5. LT Signaling Is Required for SCS Macrophage Differentiation but Is Dispensable for LN Viral Capturing
(A–F) Representative confocal micrographs of LN sections from control (A, C, E) and LTβR-Ig-treated (B, D, F) C57BL/6 mice, stained with MAbs against CD169 (green) and SIGN-R1 (red). Panels (C) and (D) are high-magnification images of SCS regions, and (E) and (F) are high-magnification images of medullary regions. Scale bars represent 100 μm (A, B) and 20 μm (C–F). (G) Viral titers in popliteal LNs of C57BL/6, μMT, and LTβR-Ig-treated C57BL/6 mice, 30 min after footpad VSV infection. Results are representative of two experiments (n = 3–4 per experiment). Differences among means are not statistically significant.
Figure 6. LT Signaling Is Required for LN VSV Replication and IFN-I Production
(A–D) Representative confocal micrographs of LN sections from control (A, C) and LTβR-Ig-treated (B, D) C57BL/6 mice at 8 hr after footpad infection with VSV-eGFP (green). Sections were stained with MAbs against CD169 (red) and B220 (blue). Scale bars represent 150 μm (A, B) and 100 μm (C, D). (E) Viral titers in popliteal LNs of C57BL/6, μMT, and LTβR-Ig-treated C57BL/6 mice, 8 hr after footpad VSV infection. Results are representative of three experiments (n = 4 per experiment). ***p < 0.001. (F and G) Ifnb1 (F) and Ifna2 (G) mRNA levels in total RNA isolated from LNs of μMT and C57BL/6 mice that were treated with CLL or with LTβR-Ig for 1 or 2 weeks prior to infection with VSV-IND. Mice were sacrificed 8 hr p.i. Analysis was performed as indicated in Figures 1D and 1E. Graphs are representative of two experiments (n = 3–4 per experiment). ***p < 0.001; **p < 0.01; *p < 0.05.
Figure 7. B Cell-Derived LTα1β2 Is Required for Survival upon Subcutaneous VSV Infection, although Adaptive Immunity Is Dispensable
(A) Kaplan-Meier survival curves of footpad VSV-infected C57BL/6 (treated or not with LTβR-Ig), μMT, or irradiated C57BL/6 mice that were reconstituted with _Lta_–/–, B _Ltb_–/–, or T _Ltb_–/– bone marrow (BM). C57BL/6 versus B _Ltb_–/– BM → C57BL/6, p < 0.0001; C57BL/6 versus T _Ltb_–/– BM → C57BL/6, not significant; B _Ltb_–/– BM → C57BL/6 versus C57BL/6 + LTβR-Ig, not significant; B _Ltb_–/– BM → C57BL/6 versus μMT, not significant; B _Ltb_–/– BM → C57BL/6 versus _Lta_–/– BM → C57BL/6, not significant; Log-rank (Mantel-Cox) test. (B) Kaplan-Meier survival curves of C57BL/6, (n = 10) BALB/c, (n = 10), and μMT (n = 13) DHLMP2A mice that were untreated (n = 11) or treated with CLL alone (n = 11), or Thy1 antibody alone (n = 15) or with CLL and Thy1 antibody (n = 15) prior to footpad VSV infection. BALB/c versus DHLMP2A + α-Thy1, not significant; BALB/c versus DHLMP2A + α-Thy1 + CLL, p < 0.0001; DHLMP2A + α-Thy1 + CLL versus μMT, not significant; DHLMP2A + α-Thy1 + CLL versus DHLMP2A + CLL, not significant; Log-rank (Mantel-Cox) test.
Comment in
- B cells: An innate talent uncovered.
Bordon Y. Bordon Y. Nat Rev Immunol. 2012 Mar 22;12(4):228-9. doi: 10.1038/nri3200. Nat Rev Immunol. 2012. PMID: 22437927 No abstract available. - B cells, not just for antibody anymore.
Khanna KM, Lefrançois L. Khanna KM, et al. Immunity. 2012 Mar 23;36(3):315-7. doi: 10.1016/j.immuni.2012.02.011. Immunity. 2012. PMID: 22444627 Free PMC article.
Similar articles
- Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus.
Iannacone M, Moseman EA, Tonti E, Bosurgi L, Junt T, Henrickson SE, Whelan SP, Guidotti LG, von Andrian UH. Iannacone M, et al. Nature. 2010 Jun 24;465(7301):1079-83. doi: 10.1038/nature09118. Nature. 2010. PMID: 20577213 Free PMC article. - Tumor Necrosis Factor-Mediated Survival of CD169+ Cells Promotes Immune Activation during Vesicular Stomatitis Virus Infection.
Shinde PV, Xu HC, Maney SK, Kloetgen A, Namineni S, Zhuang Y, Honke N, Shaabani N, Bellora N, Doerrenberg M, Trilling M, Pozdeev VI, van Rooijen N, Scheu S, Pfeffer K, Crocker PR, Tanaka M, Duggimpudi S, Knolle P, Heikenwalder M, Ruland J, Mak TW, Brenner D, Pandyra AA, Hoell JI, Borkhardt A, Häussinger D, Lang KS, Lang PA. Shinde PV, et al. J Virol. 2018 Jan 17;92(3):e01637-17. doi: 10.1128/JVI.01637-17. Print 2018 Feb 1. J Virol. 2018. PMID: 29142134 Free PMC article. - Deficiency of the B cell-activating factor receptor results in limited CD169+ macrophage function during viral infection.
Xu HC, Huang J, Khairnar V, Duhan V, Pandyra AA, Grusdat M, Shinde P, McIlwain DR, Maney SK, Gommerman J, Löhning M, Ohashi PS, Mak TW, Pieper K, Sic H, Speletas M, Eibel H, Ware CF, Tumanov AV, Kruglov AA, Nedospasov SA, Häussinger D, Recher M, Lang KS, Lang PA. Xu HC, et al. J Virol. 2015 May;89(9):4748-59. doi: 10.1128/JVI.02976-14. Epub 2015 Feb 11. J Virol. 2015. PMID: 25673724 Free PMC article. - Subcapsular Sinus Macrophages: The Seat of Innate and Adaptive Memory in Murine Lymph Nodes.
Moran I, Grootveld AK, Nguyen A, Phan TG. Moran I, et al. Trends Immunol. 2019 Jan;40(1):35-48. doi: 10.1016/j.it.2018.11.004. Epub 2018 Nov 27. Trends Immunol. 2019. PMID: 30502023 Review. - Lymph Node Subcapsular Sinus Macrophages as the Frontline of Lymphatic Immune Defense.
Louie DAP, Liao S. Louie DAP, et al. Front Immunol. 2019 Feb 28;10:347. doi: 10.3389/fimmu.2019.00347. eCollection 2019. Front Immunol. 2019. PMID: 30891035 Free PMC article. Review.
Cited by
- Activation of B cells by non-canonical helper signals.
Cerutti A, Cols M, Puga I. Cerutti A, et al. EMBO Rep. 2012 Sep;13(9):798-810. doi: 10.1038/embor.2012.111. Epub 2012 Aug 7. EMBO Rep. 2012. PMID: 22868664 Free PMC article. Review. - Pathogen manipulation of B cells: the best defence is a good offence.
Nothelfer K, Sansonetti PJ, Phalipon A. Nothelfer K, et al. Nat Rev Microbiol. 2015 Mar;13(3):173-84. doi: 10.1038/nrmicro3415. Epub 2015 Feb 9. Nat Rev Microbiol. 2015. PMID: 25659322 Review. - Macrophage heterogeneity in lymphoid tissues.
den Haan JM, Martinez-Pomares L. den Haan JM, et al. Semin Immunopathol. 2013 Sep;35(5):541-52. doi: 10.1007/s00281-013-0378-4. Epub 2013 Apr 12. Semin Immunopathol. 2013. PMID: 23579230 - IL-18 and Subcapsular Lymph Node Macrophages are Essential for Enhanced B Cell Responses with TLR4 Agonist Adjuvants.
Desbien AL, Dubois Cauwelaert N, Reed SJ, Bailor HR, Liang H, Carter D, Duthie MS, Fox CB, Reed SG, Orr MT. Desbien AL, et al. J Immunol. 2016 Dec 1;197(11):4351-4359. doi: 10.4049/jimmunol.1600993. Epub 2016 Oct 28. J Immunol. 2016. PMID: 27794001 Free PMC article. - Mechanisms of lymphatic system-specific viral replication and its potential role in autoimmune disease.
Friedrich SK, Lang PA, Friebus-Kardash J, Duhan V, Bezgovsek J, Lang KS. Friedrich SK, et al. Clin Exp Immunol. 2019 Jan;195(1):64-73. doi: 10.1111/cei.13241. Clin Exp Immunol. 2019. PMID: 30444956 Free PMC article. Review.
References
- Agyekum S, Church A, Sohail M, Krausz T, Van Noorden S, Polak J, Cohen J. Expression of lymphotoxin-beta (LT-beta) in chronic inflammatory conditions. J. Pathol. 2003;199:115–121. - PubMed
- Rabies vaccine failures. Lancet. 1988;1:917–918. Anonymous. - PubMed
- Bach P, Kamphuis E, Odermatt B, Sutter G, Buchholz CJ, Kalinke U. Vesicular stomatitis virus glycoprotein displaying retrovirus-like particles induce a type I IFN receptor-dependent switch to neutralizing IgG antibodies. J. Immunol. 2007;178:5839–5847. - PubMed
- Bachmann MF, Kündig TM, Hengartner H, Zinkernagel RM. Regulation of IgG antibody titers by the amount persisting of immune-complexed antigen. Eur. J. Immunol. 1994;24:2567–2570. - PubMed
- Bachmann MF, Hengartner H, Zinkernagel RM. T helper cell-independent neutralizing B cell response against vesicular stomatitis virus: role of antigen patterns in B cell induction? Eur. J. Immunol. 1995;25:3445–3451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA071932/CA/NCI NIH HHS/United States
- AI078897/AI/NIAID NIH HHS/United States
- 5T32-HL07623-20/HL/NHLBI NIH HHS/United States
- R01 AI069259-08/AI/NIAID NIH HHS/United States
- P01 AI078897/AI/NIAID NIH HHS/United States
- AI069259/AI/NIAID NIH HHS/United States
- T32 HL007623/HL/NHLBI NIH HHS/United States
- AI072252/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- P01 AI078897-05/AI/NIAID NIH HHS/United States
- R01 AI072252/AI/NIAID NIH HHS/United States
- R01 AI069259/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases