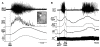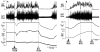Central control of brown adipose tissue thermogenesis - PubMed (original) (raw)
Central control of brown adipose tissue thermogenesis
Shaun F Morrison et al. Front Endocrinol (Lausanne). 2012.
Abstract
Thermogenesis, the production of heat energy, is an essential component of the homeostatic repertoire to maintain body temperature during the challenge of low environmental temperature and plays a key role in elevating body temperature during the febrile response to infection. Mitochondrial oxidation in brown adipose tissue (BAT) is a significant source of neurally regulated metabolic heat production in many species from mouse to man. BAT thermogenesis is regulated by neural networks in the central nervous system which responds to feedforward afferent signals from cutaneous and core body thermoreceptors and to feedback signals from brain thermosensitive neurons to activate BAT sympathetic nerve activity. This review summarizes the research leading to a model of the feedforward reflex pathway through which environmental cold stimulates BAT thermogenesis and includes the influence on this thermoregulatory network of the pyrogenic mediator, prostaglandin E(2), to increase body temperature during fever. The cold thermal afferent circuit from cutaneous thermal receptors, through second-order thermosensory neurons in the dorsal horn of the spinal cord ascends to activate neurons in the lateral parabrachial nucleus which drive GABAergic interneurons in the preoptic area (POA) to inhibit warm-sensitive, inhibitory output neurons of the POA. The resulting disinhibition of BAT thermogenesis-promoting neurons in the dorsomedial hypothalamus activates BAT sympathetic premotor neurons in the rostral ventromedial medulla, including the rostral raphe pallidus, which provide excitatory, and possibly disinhibitory, inputs to spinal sympathetic circuits to drive BAT thermogenesis. Other recently recognized central sites influencing BAT thermogenesis and energy expenditure are also described.
Figures
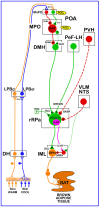
Figure 1
Schematic of the proposed neuroanatomical and neurotransmitter model for the core thermoregulatory network and other CNS sites controlling brown adipose tissue (BAT) thermogenesis. Cool and warm cutaneous thermal sensory receptors transmit signals to respective primary sensory neurons in the dorsal root ganglia which relay this thermal information to second-order thermal sensory neurons in the dorsal horn (DH). Cool sensory DH neurons glutamatergically activate third-order sensory neurons in the external lateral subnucleus of the lateral parabrachial nucleus (LPBel), while warm sensory DH neurons project to third-order sensory neurons in the dorsal subnucleus of the lateral parabrachial nucleus (LPBd). Thermosensory signals for thermoregulatory responses are transmitted from the LPB to the preoptic area (POA) where GABAergic interneurons in the median preoptic (MnPO) subnucleus are activated by glutamatergic inputs from cool-activated neurons in LPBel and inhibit a BAT-regulating population of warm-sensitive (W-S) neurons in the medial preoptic (MPO) subnucleus. In contrast, glutamatergic interneurons in the MnPO, postulated to be excited by glutamatergic inputs from warm-activated neurons in LPBd, excite W-S neurons in MPO. Prostaglandin (PG) E2 binds to EP3 receptors to inhibit the activity of W-S neurons in the POA. Preoptic W-S neurons providing thermoregulatory control of BAT thermogenesis inhibit BAT sympathoexcitatory neurons in the dorsomedial hypothalamus (DMH) which, when disinhibited during skin cooling, excite BAT sympathetic premotor neurons in the rostral ventromedial medulla, including the rostral raphe pallidus (rRPa), that project to BAT sympathetic preganglionic neurons (SPN) in the spinal intermediolateral nucleus (IML). Some BAT premotor neurons can release glutamate (GLU) to excite BAT SPNs and increase BAT sympathetic nerve activity, while others can release serotonin (5-HT) to interact with 5-HT1A receptors, potentially on inhibitory interneurons in the IML, to increase the BAT sympathetic outflow and thermogenesis. Orexinergic neurons in the perifornical lateral hypothalamus (PeF-LH) project to the rRPa to increase the excitability of BAT sympathetic premotor neurons. Neurons in the paraventricular hypothalamic (PVH) nucleus exert an inhibitory influence on BAT thermogenesis, possibly via a GABAergic input to BAT sympathetic premotor neurons in rRPa. Activation of neurons in the ventrolateral medulla (VLM) or in the nucleus of the solitary tract (NTS) produces an inhibition of BAT thermogenesis, potentially via a non-GABAergic input to rRPa or by activation of spinal inhibitory interneurons in the IML. VGLUT3, vesicular glutamate transporter 3.
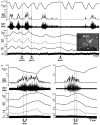
Figure 2
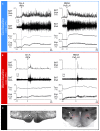
Inhibition of neurons in the median preoptic nucleus (MnPO) or blockade of GABAA receptors in the medial preoptic nucleus (MPO) prevents skin cooling-evoked BAT thermogenesis. (A) Before and after injection of saline (SAL) vehicle into the MnPO [inset: typical injection site (arrow) in the MnPO; 3v, third ventricle; ox, optic chiasm; ac, anterior commissure], episodes of skin cooling evoke increases in BAT sympathetic nerve activity (SNA), BAT temperature (TBAT), expired CO2 (Exp CO2), and heart rate (HR), with no change in arterial pressure (AP). Following nanoinjection of the inhibitory transmitter, glycine (GLY), into the MnPO, skin cooling no longer increases these thermoregulatory parameters. Modified with permission from Nakamura and Morrison (2008a). (B) The skin cooling-evoked increases in thermoregulatory parameters, including BAT SNA and TBAT, are unaffected by nanoinjection of saline vehicle into the MPO, but these increases are reversed by blockade of GABAA receptors in MPO with nanoinjection of bicuculline (BIC). Modified with permission from Nakamura and Morrison (2007).
Figure 3
Disinhibition of neurons in the dorsomedial hypothalamus (DMH) increases brown adipose tissue (BAT) thermogenesis and inhibition of neurons in the DMH reverses febrile-evoked BAT sympathetic nerve activity (SNA) and thermogenesis. (A) Nanoinjection of the GABAA antagonist, bicuculline (BIC), into the DMH, (white arrowhead in inset histological section through the DMH) increased BAT SNA, BAT temperature (BAT Temp), and expired CO2. Modified with permission from Morrison et al. (2004). (B) Nanoinjection of prostaglandin E2 (PGE2) into the medial preoptic area (MPO) increased BAT SNA, BAT Temp, Exp CO2, heart rate (HR), and arterial pressure (AP). Subsequent bilateral nanoinjection of the glutamate receptor antagonist, kynurenate (KYN), into the DMH completely reversed these PGE2-evoked responses. Modified with permission from Madden and Morrison (2004).
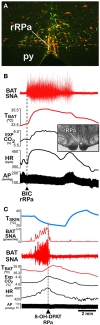
Figure 4
Effects of disinhibition and inhibition of rostral raphe pallidus (rRPa) neurons on brown adipose tissue (BAT) thermogenesis. (A) Coronal histological section through the rostral medulla at the level of the facial nucleus and the rRPa, containing immunohistochemically labeled neurons that were trans-synaptically infected following inoculations of BAT with pseudorabies virus (red), that contain the serotonin synthesizing enzyme, tryptophan hydroxylase (green), or that contain both markers (yellow). Py, pyramidal tract. Modified with permission from Cano et al. (2003). (B) Disinhibition of neurons in the rRPa [arrowhead in inset histological section through rRPa at the level of the facial nucleus (7n)] with nanoinjections of bicuculline (BIC) elicits dramatic increases in BAT sympathetic nerve activity (SNA), BAT temperature (TBAT), expired CO2 (Exp CO2), and heart rate (HR), with little change ion arterial pressure (AP). Modified with permission from Madden and Morrison (2003). (C) Inhibition of local neurons in the rRPa with a nanoinjection of the inhibitory serotonin 1A receptor agonist, 8-OH-DPAT, produces a rapid and complete reversal of the skin cooling-evoked increases in BAT SNA and an immediate waning of the accompanying metabolic and cardiac responses, despite the sustained reduction in skin temperature (TSKIN). Modified with permission from Nakamura and Morrison (2007).
Figure 5
Orexin in the rostral raphe pallidus (rRPa) or activation of neurons in the perifornical lateral hypothalamus (PeF-LH) produces a prolonged increase in BAT sympathetic nerve activity (SNA) and BAT thermogenesis in cool, but not warm rats. (A) Under cool conditions (core temperature <37°C) in which a low level of basal BAT SNA is present, nanoinjections of orexin-A (Orx-A, left panel, dashed line) in the rRPa or of N-methyl-D-aspartate (NMDA, right panel, dashed line) into the PeF-LH elicited prolonged increases in BAT SNA, BAT temperature (TBAT), and expired CO2 (Exp CO2). **(B)** Under warm conditions (core temperature >37°C) in which no basal BAT SNA was present, nanoinjections of Orx-A (left panel, dashed line) in rRPa or of NMDA (right panel, dashed line) into the PeF-LH failed to evoke significant changes in BAT SNA, TBAT or Exp CO2. (C) Representative histological sections illustrating nanoinjection sites in the rRPa (left panel, white arrowhead) and in the PeF-LH (right panel, white arrowhead). Note that the NMDA injection sites in the PeF-LH were located in the midst of many neurons immunohistochemically labeled for Orexin-A (red). Modified with permission from Tupone et al. (2011).
Figure 6
Disinhibition of neurons in the paraventricular hypothalamic (PVH) nucleus inhibits the increases in BAT sympathetic nerve activity (SNA) evoked by cooling, but not those evoked by disinhibition of neurons in the rostral raphe pallidus (rRPa). (A) Nanoinjection of bicuculline (BIC) into the PVH completely reversed the increases in BAT SNA, BAT temperature (TBAT), expired CO2 (Exp CO2) produced by whole body cooling. Core body temperature (TCORE) was also reduced; heart rate (HR) and arterial pressure (AP) were increased by BIC in PVH. (B) The increases in BAT SNA, TBAT, expired CO2, TCORE, HR, and AP evoked by nanoinjection of BIC into the rRPa are not affected by nanoinjection of BIC into the PVH. Modified with permission from Madden and Morrison (2009).
Figure 7
Activation of neurons in the ventrolateral medulla (VLM) or in the nucleus of the solitary tract (NTS) inhibits BAT sympathetic nerve activity (SNA) and BAT thermogenesis. (A) Cooling-evoked increases in BAT SNA, BAT temperature (TBAT), and expired CO2 (Exp CO2) were reversed following unilateral nanoinjection of either the glutamate receptor agonist, NMDA, or the GABAA receptor antagonist, bicuculline (BIC), into the rostral VLM (RVLM). Core temperature (TCORE) was also reduced by activation of RVLM neurons. (B) Increases in BAT SNA, TBAT, and ExpCO2 evoked by BIC nanoinjection into the rostral raphe pallidus (rRPa) were reversed by bilateral nanoinjections of BIC into the medial NTS. Modified with permission from Cao et al. (2010).
Similar articles
- Central control of thermogenesis in mammals.
Morrison SF, Nakamura K, Madden CJ. Morrison SF, et al. Exp Physiol. 2008 Jul;93(7):773-97. doi: 10.1113/expphysiol.2007.041848. Epub 2008 May 9. Exp Physiol. 2008. PMID: 18469069 Free PMC article. Review. - Central circuitries for body temperature regulation and fever.
Nakamura K. Nakamura K. Am J Physiol Regul Integr Comp Physiol. 2011 Nov;301(5):R1207-28. doi: 10.1152/ajpregu.00109.2011. Epub 2011 Sep 7. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21900642 Review. - Central neural control of thermoregulation and brown adipose tissue.
Morrison SF. Morrison SF. Auton Neurosci. 2016 Apr;196:14-24. doi: 10.1016/j.autneu.2016.02.010. Epub 2016 Feb 23. Auton Neurosci. 2016. PMID: 26924538 Free PMC article. Review. - 2010 Carl Ludwig Distinguished Lectureship of the APS Neural Control and Autonomic Regulation Section: Central neural pathways for thermoregulatory cold defense.
Morrison SF. Morrison SF. J Appl Physiol (1985). 2011 May;110(5):1137-49. doi: 10.1152/japplphysiol.01227.2010. Epub 2011 Jan 26. J Appl Physiol (1985). 2011. PMID: 21270352 Free PMC article. - Central neural pathways for thermoregulation.
Morrison SF, Nakamura K. Morrison SF, et al. Front Biosci (Landmark Ed). 2011 Jan 1;16(1):74-104. doi: 10.2741/3677. Front Biosci (Landmark Ed). 2011. PMID: 21196160 Free PMC article. Review.
Cited by
- Adipose Tissue-Derived Extracellular Vesicles: A Promising Biomarker and Therapeutic Strategy for Metabolic Disorders.
Liu W, Liu T, Zhao Q, Ma J, Jiang J, Shi H. Liu W, et al. Stem Cells Int. 2023 Dec 26;2023:9517826. doi: 10.1155/2023/9517826. eCollection 2023. Stem Cells Int. 2023. PMID: 38169960 Free PMC article. Review. - The Role of Proton-Coupled Amino Acid Transporter 2 (SLC36A2) in Cold-Induced Thermogenesis of Mice.
Shu H, Zhang J, Cheng D, Zhao X, Ma Y, Zhang C, Zhang Y, Jia Z, Liu Z. Shu H, et al. Nutrients. 2023 Aug 11;15(16):3552. doi: 10.3390/nu15163552. Nutrients. 2023. PMID: 37630739 Free PMC article. - Browning of Adipocytes: A Potential Therapeutic Approach to Obesity.
Schirinzi V, Poli C, Berteotti C, Leone A. Schirinzi V, et al. Nutrients. 2023 May 8;15(9):2229. doi: 10.3390/nu15092229. Nutrients. 2023. PMID: 37432449 Free PMC article. Review. - Increasing adipocyte number and reducing adipocyte size: the role of retinoids in adipose tissue development and metabolism.
Wang B, Du M. Wang B, et al. Crit Rev Food Sci Nutr. 2024;64(29):10608-10625. doi: 10.1080/10408398.2023.2227258. Epub 2023 Jul 10. Crit Rev Food Sci Nutr. 2024. PMID: 37427553 Review. - TLR4 in POMC neurons regulates thermogenesis in a sex-dependent manner.
Li Y, Zhu S, Du D, Li Q, Xie K, Chen L, Feng X, Wu X, Sun Z, Zhou J, Yang J, Shu G, Wang S, Gao P, Zhu C, Jiang Q, Wang L. Li Y, et al. J Lipid Res. 2023 May;64(5):100368. doi: 10.1016/j.jlr.2023.100368. Epub 2023 Apr 6. J Lipid Res. 2023. PMID: 37028769 Free PMC article.
References
- Amini-Sereshki L., Zarrindast M. R. (1984). Brain stem tonic inhibition of thermoregulation in the rat. Am. J. Physiol. 247, R154–R159 - PubMed
- Bacon S. J., Smith A. D. (1988). Preganglionic sympathetic neurones innervating the rat adrenal medulla: immunocytochemical evidence of synaptic input from nerve terminals containing substance P, GABA or 5-hydroxytryptamine. J. Auton. Nerv. Syst. 24, 97–12210.1016/0165-1838(88)90140-3 - DOI - PubMed
- Balthasar N., Dalgaard L. T., Lee C. E., Yu J., Funahashi H., Williams T., Ferreira M., Tang V., Mcgovern R. A., Kenny C. D., Christiansen L. M., Edelstein E., Choi B., Boss O., Aschkenasi C., Zhang C. Y., Mountjoy K., Kishi T., Elmquist J. K., Lowell B. B. (2005). Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123, 493–50510.1016/j.cell.2005.08.035 - DOI - PubMed
Grants and funding
- F32 DK065401/DK/NIDDK NIH HHS/United States
- R01 NS040987-01A1/NS/NINDS NIH HHS/United States
- R56 DK082558-01A1/DK/NIDDK NIH HHS/United States
- R01 NS040987/NS/NINDS NIH HHS/United States
- R01 DK057838-01A1/DK/NIDDK NIH HHS/United States
- R56 DK082558/DK/NIDDK NIH HHS/United States
- R01 DK057838/DK/NIDDK NIH HHS/United States
- F32 DK065401-03/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources