Injury-independent induction of reactive gliosis in retina by loss of function of the LIM homeodomain transcription factor Lhx2 - PubMed (original) (raw)
Injury-independent induction of reactive gliosis in retina by loss of function of the LIM homeodomain transcription factor Lhx2
Jimmy de Melo et al. Proc Natl Acad Sci U S A. 2012.
Abstract
Müller glia are the primary glial subtype in the retina and perform a wide range of physiological tasks in support of retinal function, but little is known about the transcriptional network that maintains these cells in their differentiated state. We report that selective deletion of the LIM homeodomain transcription factor Lhx2 from mature Müller glia leads to the induction of reactive retinal gliosis in the absence of injury. Furthermore, Lhx2 expression is also down-regulated in Prph2(Rd2/Rd2) animals immediately before the onset of reactive gliosis. Analysis of conditional Lhx2 knockouts showed that gliosis was hypertrophic but not proliferative. Aging of experimental animals demonstrated that constitutive reactive gliosis induced by deletion of Lhx2 reduced rates of ongoing apoptosis and compromised both rod and cone photoreceptor function. Additionally, these animals showed a dramatically reduced ability to induce expression of secreted neuroprotective factors and displayed enhanced rates of apoptosis in light-damage assays. We provide in vivo evidence that Lhx2 actively maintains mature Müller glia in a nonreactive state, with loss of function initiating a specific program of nonproliferative hypertrophic gliosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
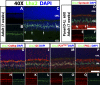
Expression of Lhx2 in the adult (7-wk-old) mouse retina. (A_–_C) Lhx2 is expressed in the medial INL in the adult retina. A small subpopulation of Lhx2-positive cells is localized to the inner INL (C, arrows). (D_–_F) Cells in the inner INL coexpressed the pan-amacrine cell marker syntaxin (D, arrows). (G_–_O) The overwhelming majority of Lhx2-positive cells in the medial INL coexpressed the Müller glia markers Cralbp, P27Kip1, and GS. (P_–_R) These cells did not express PKCα, a marker of bipolar interneurons. (Scale bars: 50 μm.)
Fig. 2.
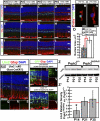
GLAST-CreERT2 specifically and efficiently excises Lhx2 in Müller glia after induction with 4-OHT. (A) Schema for generation of Lhx2 ΔcKO mice. (B_–_D) Prospective labeling with ROSA26stopYFP;GLAST-CreERT2 confirms the Müller glial specificity of the GLAST-CreERT2 transgene. (E_–_J) Lhx2 expression is eliminated in GLAST-CreERT2;Lhx2lox/lox (Lhx2 ΔcKO) but not GLAST-CreERT2;Lhx2lox/+ (Lhx2 ΔcKO/+) animals. A small population of cells continues to express Lhx2 (J, arrows). (K) Lhx2 ΔKO animals display a significant reduction in Lhx2 expression at P26 and P55 (79%, P < 0.005, n = 3; 83%, P < 0.005, n = 3, respectively). There was no significant reduction of cells expressing the Müller glial marker P27Kip1. (L) The majority of remaining Lhx2-positive cells are Müller glia (P26: 89.3 ± 3.5% P27Kip1-positive, 93.1 ± 1.2% Cralbp-positive; P55: 91.8 ± 1.7% P27Kip1-positive, 92.1 ± 0.9% Cralbp-positive; all n = 3). (M_–_P) Expression of the Müller glial markers P27Kip1 and Cralbp are not qualitatively altered in Lhx2 ΔcKO animals. (Scale bars: 50 μm.)
Fig. 3.
Lhx2 ΔcKO mice display progressive and ubiquitous hypertrophic reactive gliosis of Müller glia. (A_–_H) Expression of the neurofilament elements Gfap and vimentin are up-regulated in Lhx2 ΔcKO mice by P55. (A and B) Vimentin expression is unchanged at P26 immediately after 4-OHT treatment. (C and D) By P55, expression of vimentin is up-regulated in Lhx2 ΔcKO mice. (E and F) Gfap expression is unchanged at P26 immediately after 4-OHT treatment. (G and H) By P55, expression of Gfap is up-regulated in Lhx2 ΔcKO mice. (I_–_L) Expression of GS is down-regulated in Lhx2 ΔcKO mice at P26 and P55. (M and N) Lhx2 ΔcKO Müller glia display nuclear hypertrophy. (O) Nuclei of Lhx2 ΔcKO cells are 43% larger than Lhx2-positive nuclei (66.98 μm2 vs. 37.94889 μm2, P < 0.0001, _n_ = 27). (_P_–_S_) Generation of mosaic retinas of Lhx2-positive and Lhx2-negative Müller glia demonstrates reduced reactive gliosis associated with Lhx2-positive Müller glia clusters (_P_, between white dashed lines). (_T_–_Y_) Further mosaics were generated by electroporation of Cre recombinase (pCAG-Cre) expression constructs into P0.5 Lhx2lox/lox and Lhx2lox/+ mice. By P14, induction of reactive gliosis was specifically restricted to the electroporated regions in Lhx2lox/lox (_T_–_V_; _T_, dashed line) but not Lhx2lox/+ (_W_–_Y_) mice. (_Z_) Down-regulation of Lhx2 at P14 precedes induction of reactive gliosis in Prph2Rd2/Rd2 mice. (_Z_′) Relative density of Lhx2 at P14, P21, and P35 normalized to Gapdh demonstrates significant 52% reduction of Lhx2 at P14 (_P_ < 0.02, _n_ = 3). There is no significant difference in Lhx2 relative density at P21 or P35 (_P_ > 0.05, n = 3). (Scale bars: A_–_L, P, T, and W, 50 μm; M and N, 10 μm.)
Fig. 4.
Lhx2 ΔcKO Müller glia display hallmarks of reactive gliosis at 1 y after 4-OHT treatment. (A_–_E) Lhx2 ΔcKO retinas display ubiquitous up-regulation of Gfap and β-catenin expression in Müller glia at 1 y after 4-OHT treatment. (C_–_E) Outer limiting membrane breaks are evident in these animals because of Müller glia hypertrophy (arrows). (F) Retinas with reactive gliosis generated by Lhx2 ΔcKO feature significantly fewer TUNEL-positive apoptotic cells than age matched controls do at P55 (P < 0.05, n = 3), 3 mo (P < 0.05, n = 3), and 1 y (P < 0.05, n = 3) after 4-OHT treatment. (Scale bars: 50 μm.)
Fig. 5.
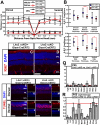
Lhx2 ΔcKO mice demonstrate a decreased capacity to protect photoreceptors in light-damage assays. (A) Plot of Lhx2 ΔcKO and Lhx2 ΔcKO/+ retinal and ONL thickness after 6,000-lx light exposure. (B) Statistical comparison of overall, dorsal, and ventral retinal and ONL thickness by using generalized estimating equations (G.E.E.). Plots display the G.E.E. coefficient and 95% confidence intervals. (A and B) Overall retinal thickness was not significantly different between Lhx2 ΔcKO and Lhx2 ΔcKO/+ mice (P > 0.05, n = 3) because of the occasional formation of ventral dysplasia in the inner retina of Lhx2 ΔcKO mice. Retinal thickness was significantly different in the dorsal retina (P < 0.005, n = 3). Overall, dorsal, and ventral ONL thickness was significantly reduced in the Lhx2 ΔcKO mice (P < 0.005, n = 3 each of overall, dorsal, and ventral). (C and D) Lhx2 ΔcKO and Lhx2 ΔcKO/+ retinas do not display cell proliferation after light damage. (E_–_P) Lhx2 ΔcKO retinas feature increased TUNEL staining after light exposure in both the dorsal (E_–_G and K_–_M) and ventral (H_–_J and N_–_P) retina compared with Lhx2 ΔcKO/+ mice. (Q and R) Relative target gene expression in real-time PCR analysis of P55 Lhx2 ΔcKO vs. Lhx2 ΔcKO/+ mice (Q) and P55 Lhx2 ΔcKO vs. Lhx2 ΔcKO/+ mice subjected to light damage (R). For all targets, Gapdh was used as an endogenous control gene. (Scale bars: 50 μm.)
Similar articles
- Lhx2 Is an Essential Factor for Retinal Gliogenesis and Notch Signaling.
de Melo J, Zibetti C, Clark BS, Hwang W, Miranda-Angulo AL, Qian J, Blackshaw S. de Melo J, et al. J Neurosci. 2016 Feb 24;36(8):2391-405. doi: 10.1523/JNEUROSCI.3145-15.2016. J Neurosci. 2016. PMID: 26911688 Free PMC article. - Lhx2 balances progenitor maintenance with neurogenic output and promotes competence state progression in the developing retina.
Gordon PJ, Yun S, Clark AM, Monuki ES, Murtaugh LC, Levine EM. Gordon PJ, et al. J Neurosci. 2013 Jul 24;33(30):12197-207. doi: 10.1523/JNEUROSCI.1494-13.2013. J Neurosci. 2013. PMID: 23884928 Free PMC article. - Multiple intrinsic factors act in concert with Lhx2 to direct retinal gliogenesis.
de Melo J, Clark BS, Blackshaw S. de Melo J, et al. Sci Rep. 2016 Sep 8;6:32757. doi: 10.1038/srep32757. Sci Rep. 2016. PMID: 27605455 Free PMC article. - Lhx2, an evolutionarily conserved, multifunctional regulator of forebrain development.
Chou SJ, Tole S. Chou SJ, et al. Brain Res. 2019 Feb 15;1705:1-14. doi: 10.1016/j.brainres.2018.02.046. Epub 2018 Mar 6. Brain Res. 2019. PMID: 29522720 Review. - Müller Glia Reactivity and Development of Gliosis in Response to Pathological Conditions.
Graca AB, Hippert C, Pearson RA. Graca AB, et al. Adv Exp Med Biol. 2018;1074:303-308. doi: 10.1007/978-3-319-75402-4_37. Adv Exp Med Biol. 2018. PMID: 29721957 Review.
Cited by
- Suppression of inner blood-retinal barrier breakdown and pathogenic Müller glia activation in ischemia retinopathy by myeloid cell depletion.
Zhou L, Xu Z, Lu H, Cho H, Xie Y, Lee G, Ri K, Duh EJ. Zhou L, et al. J Neuroinflammation. 2024 Aug 24;21(1):210. doi: 10.1186/s12974-024-03190-9. J Neuroinflammation. 2024. PMID: 39182142 Free PMC article. - Regulation of Spiral Ganglion Neuron Regeneration as a Therapeutic Strategy in Sensorineural Hearing Loss.
Wang M, Xu L, Han Y, Wang X, Chen F, Lu J, Wang H, Liu W. Wang M, et al. Front Mol Neurosci. 2022 Jan 20;14:829564. doi: 10.3389/fnmol.2021.829564. eCollection 2021. Front Mol Neurosci. 2022. PMID: 35126054 Free PMC article. Review. - Transcription factor SOX9 plays a key role in the regulation of visual cycle gene expression in the retinal pigment epithelium.
Masuda T, Wahlin K, Wan J, Hu J, Maruotti J, Yang X, Iacovelli J, Wolkow N, Kist R, Dunaief JL, Qian J, Zack DJ, Esumi N. Masuda T, et al. J Biol Chem. 2014 May 2;289(18):12908-21. doi: 10.1074/jbc.M114.556738. Epub 2014 Mar 14. J Biol Chem. 2014. PMID: 24634209 Free PMC article. - The LIM homeodomain factor Lhx2 is required for hypothalamic tanycyte specification and differentiation.
Salvatierra J, Lee DA, Zibetti C, Duran-Moreno M, Yoo S, Newman EA, Wang H, Bedont JL, de Melo J, Miranda-Angulo AL, Gil-Perotin S, Garcia-Verdugo JM, Blackshaw S. Salvatierra J, et al. J Neurosci. 2014 Dec 10;34(50):16809-20. doi: 10.1523/JNEUROSCI.1711-14.2014. J Neurosci. 2014. PMID: 25505333 Free PMC article. - Characteristics of blood-brain barrier heterogeneity between brain regions revealed by profiling vascular and perivascular cells.
Pfau SJ, Langen UH, Fisher TM, Prakash I, Nagpurwala F, Lozoya RA, Lee WA, Wu Z, Gu C. Pfau SJ, et al. Nat Neurosci. 2024 Oct;27(10):1892-1903. doi: 10.1038/s41593-024-01743-y. Epub 2024 Aug 29. Nat Neurosci. 2024. PMID: 39210068 Free PMC article.
References
- Bringmann A, et al. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25:397–424. - PubMed
- Bringmann A, et al. Role of retinal glial cells in neurotransmitter uptake and metabolism. Neurochem Int. 2009;54(3-4):143–160. - PubMed
- Furukawa T, Mukherjee S, Bao ZZ, Morrow EM, Cepko CL. rax, Hes1, and notch1 promote the formation of Müller glia by postnatal retinal progenitor cells. Neuron. 2000;26:383–394. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous