Ubiquitination-mediated regulation of interferon responses - PubMed (original) (raw)
Review
Ubiquitination-mediated regulation of interferon responses
Serge Y Fuchs. Growth Factors. 2012 Jun.
Abstract
Interferon cytokine family members shape the immune response to protect the host from both pathologic infections and tumorigenesis. To mediate their physiologic function, interferons evoke a robust and complex signal transduction pathway that leads to the induction of interferon-stimulated genes with both proinflammatory and antiviral functions. Numerous mechanisms exist to tightly regulate the extent and duration of these cellular responses. Among such mechanisms, the post-translational conjugation of ubiquitin polypeptides to protein mediators of interferon signaling has emerged as a crucially important mode of control. In this mini-review, we highlight recent advances in our understanding of these ubiquitin-mediated mechanisms, their exploitation by invading viruses, and their possible utilization for medical intervention.
Figures
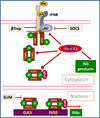
Figure 1. IFN signaling and the regulatory role of ubiquitination
Upon engaging a cognate receptor complex (IFNAR1-IFNAR2 for Type 1 IFN, IFNGR1-IFNGR2 for Type 2 IFN or IL28RA-IL10R2 for Type 3 IFN) IFN activate receptor-associated JAKs. A resulting tyrosine phosphorylation of STAT proteins leads to the formation of transcriptionally active complexes (STAT1 homodimers for all types and additional STAT1-STAT2-IRF9 complexes for Types 1/3 IFN) and ensuing induction of ISGs. Cellular (β-Trcp, SOCS, SLIM) and diverse viral E3 ubiquitin ligases facilitate the ubiquitination of various mediators of IFN signaling pathway to limit the extent and duration of this signaling.
Figure 2. A hypothetical mechanism for ubiquitination-mediated stimulation of IFNAR1 internalization
Sequence alignment of membrane-adjacent proximal fragments of the cytoplasmic tail of IFNAR1 is shown on the left. Tyr-based endocytic motif YXXΨ, where Ψ is a hydrophobic residue, is shown in bold letters. Proline residues conferring the IFNAR1 tail turns are marked by green letters. Figure also shows an unstimulated receptor whose endocytic motif does not interact with endocytic machinery (e.g., AP50 protein) due to spatial interference by associated TYK2. Upon ligand binding and ensuing ubiquitination, a putative conformational change can allow the recognition of the endocytic motif and subsequent internalization. We speculate that formation of two different types of polyubiquitin chains (Lys48- versus Lys63-linked) oriented toward plasma membrane at different angles may stabilize the conformation conducive for endocytosis.
Similar articles
- Viral hijacking of the host ubiquitin system to evade interferon responses.
Viswanathan K, Früh K, DeFilippis V. Viswanathan K, et al. Curr Opin Microbiol. 2010 Aug;13(4):517-23. doi: 10.1016/j.mib.2010.05.012. Epub 2010 Jun 17. Curr Opin Microbiol. 2010. PMID: 20699190 Free PMC article. Review. - Robust Lys63-Linked Ubiquitination of RIG-I Promotes Cytokine Eruption in Early Influenza B Virus Infection.
Jiang J, Li J, Fan W, Zheng W, Yu M, Chen C, Sun L, Bi Y, Ding C, Gao GF, Liu W. Jiang J, et al. J Virol. 2016 Jun 24;90(14):6263-6275. doi: 10.1128/JVI.00549-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27122586 Free PMC article. - ISG15 inhibits Nedd4 ubiquitin E3 activity and enhances the innate antiviral response.
Malakhova OA, Zhang DE. Malakhova OA, et al. J Biol Chem. 2008 Apr 4;283(14):8783-7. doi: 10.1074/jbc.C800030200. Epub 2008 Feb 20. J Biol Chem. 2008. PMID: 18287095 Free PMC article. - Negative regulation of the RLH signaling by the E3 ubiquitin ligase RNF114.
Lin B, Ke Q, Li H, Pheifer NS, Velliquette DC, Leaman DW. Lin B, et al. Cytokine. 2017 Nov;99:186-193. doi: 10.1016/j.cyto.2017.05.002. Epub 2017 Jul 29. Cytokine. 2017. PMID: 28625874 - Chain reactions: molecular mechanisms of RBR ubiquitin ligases.
Cotton TR, Lechtenberg BC. Cotton TR, et al. Biochem Soc Trans. 2020 Aug 28;48(4):1737-1750. doi: 10.1042/BST20200237. Biochem Soc Trans. 2020. PMID: 32677670 Free PMC article. Review.
Cited by
- Type I interferon controls propagation of long interspersed element-1.
Yu Q, Carbone CJ, Katlinskaya YV, Zheng H, Zheng K, Luo M, Wang PJ, Greenberg RA, Fuchs SY. Yu Q, et al. J Biol Chem. 2015 Apr 17;290(16):10191-9. doi: 10.1074/jbc.M114.612374. Epub 2015 Feb 25. J Biol Chem. 2015. PMID: 25716322 Free PMC article. - Interferon gamma receptor: the beginning of the journey.
Blouin CM, Lamaze C. Blouin CM, et al. Front Immunol. 2013 Sep 3;4:267. doi: 10.3389/fimmu.2013.00267. Front Immunol. 2013. PMID: 24027571 Free PMC article. Review. - RINCK-mediated monoubiquitination of cGAS promotes antiviral innate immune responses.
Liu ZS, Zhang ZY, Cai H, Zhao M, Mao J, Dai J, Xia T, Zhang XM, Li T. Liu ZS, et al. Cell Biosci. 2018 May 9;8:35. doi: 10.1186/s13578-018-0233-3. eCollection 2018. Cell Biosci. 2018. PMID: 29760876 Free PMC article. - A Potent In Vivo Antitumor Efficacy of Novel Recombinant Type I Interferon.
Zhang KJ, Yin XF, Yang YQ, Li HL, Xu YN, Chen LY, Liu XJ, Yuan SJ, Fang XL, Xiao J, Wu S, Xu HN, Chu L, Katlinski KV, Katlinskaya YV, Guo RB, Wei GW, Wang DC, Liu XY, Fuchs SY. Zhang KJ, et al. Clin Cancer Res. 2017 Apr 15;23(8):2038-2049. doi: 10.1158/1078-0432.CCR-16-1386. Epub 2016 Sep 28. Clin Cancer Res. 2017. PMID: 27683179 Free PMC article. - FAS-associated factor-1 positively regulates type I interferon response to RNA virus infection by targeting NLRX1.
Kim JH, Park ME, Nikapitiya C, Kim TH, Uddin MB, Lee HC, Kim E, Ma JY, Jung JU, Kim CJ, Lee JS. Kim JH, et al. PLoS Pathog. 2017 May 22;13(5):e1006398. doi: 10.1371/journal.ppat.1006398. eCollection 2017 May. PLoS Pathog. 2017. PMID: 28542569 Free PMC article.
References
- Ali S, Nouhi Z, Chughtai N. SHP-2 regulates SOCS-1-mediated Janus kinase-2 ubiquitination/degradation downstream of the prolactin receptor. J Biol Chem. 2003;278:52021–52031. - PubMed
- Bibeau-Poirier A, Servant MJ. Roles of ubiquitination in pattern-recognition receptors and type I interferon receptor signaling. Cytokine. 2008;43:359–367. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources