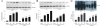Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage - PubMed (original) (raw)
Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage
Sen Lin et al. J Neuroinflammation. 2012.
Abstract
Background: Inflammatory injury plays a critical role in intracerebral hemorrhage (ICH)-induced neurological deficits; however, the signaling pathways are not apparent by which the upstream cellular events trigger innate immune and inflammatory responses that contribute to neurological impairments. Toll-like receptor 4 (TLR4) plays a role in inflammatory damage caused by brain disorders.
Methods: In this study, we investigate the role of TLR4 signaling in ICH-induced inflammation. In the ICH model, a significant upregulation of TLR4 expression in reactive microglia has been demonstrated using real-time RT-PCR. Activation of microglia was detected by immunohistochemistry, cytokines were measured by ELISA, MyD88, TRIF and NF-κB were measured by Western blot and EMSA, animal behavior was evaluated by animal behavioristics.
Results: Compared to WT mice, TLR4(-/-) mice had restrained ICH-induced brain damage showing in reduced cerebral edema and lower neurological deficit scores. Quantification of cytokines including IL-6, TNF-α and IL-1β and assessment of macrophage infiltration in perihematoma tissues from TLR4(-/-), MyD88(-/-) and TRIF(-/-) mice showed attenuated inflammatory damage after ICH. TLR4(-/-) mice also exhibited reduced MyD88 and TRIF expression which was accompanied by decreased NF-κB activity. This suggests that after ICH both MyD88 and TRIF pathways might be involved in TLR4-mediated inflammatory injury possibly via NF-κB activation. Exogenous hemin administration significantly increased TLR4 expression and microglial activation in cultures and also exacerbated brain injury in WT mice but not in TLR4(-/-) mice. Anti-TLR4 antibody administration suppressed hemin-induced microglial activation in cultures and in the mice model of ICH.
Conclusions: Our findings suggest that heme potentiates microglial activation via TLR4, in turn inducing NF-κB activation via the MyD88/TRIF signaling pathway, and ultimately increasing cytokine expression and inflammatory injury in ICH. Targeting TLR4 signaling may be a promising therapeutic strategy for ICH.
Figures
Figure 1
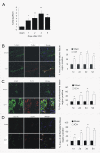
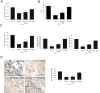
TLR4 mRNA and protein expression after ICH. A) Real-time RT-PCR shows an upregulation of TLR4 mRNA expression in perihematoma tissues in WT mice (n = 6) on days 1, 2, 3, and 5 post ICH. B) TLR4 co-labeling with tubulin-positive neuron (arrows, n = 6). C) TLR4 co-labeling with GFAP-positive astrocytes (arrows, n = 6). D) TLR4 co-labeling with CD11b-positive microglia. Compared to sham control mice (n = 6), ICH induced significant increase in TLR4 protein. *P < 0.05, **P < 0.01 vs. sham control. Values (mean ± SD) are representative of two independent experiments. Bar = 20 μM.
Figure 2
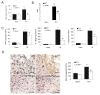
TLR4−/− mice displayed attenuated brain injury and decreased neuroinflammation. TLR4−/− had decreased brain water content (A, n = 3) and decreased NDS (B, n = 3). ELISA showed that TLR4−/− mice had a marked decrease in release of IL-6, TNF-α, and IL-1β (C, n = 3) on day 3 post-ICH. D) Immunohistochemistry of CD68 showed decreased macrophage infiltration in TLR4−/− mice on day 3 post-ICH (n = 3). **P < 0.01 vs. sham group; ##P < 0. 01 vs. WT group; Bar = 50 μM in D. Values (mean ± SD) are representative of three independent experiments.
Figure 3
Declined neurologic deficits and inflammation in MyD88 −/− and TRIF−/− mice. Compared to WT mice, both MyD88−/− and TRIF−/− mice had decreased brain water content (A), NDS (B), (C) Both MyD88−/− and TRIF−/− mice showed decreased protein levels of IL-6, TNF-α, and IL-1β, and (D) immunohistochemistry of CD68 showed decreased macrophage infiltration in the perihematoma region of MyD88−/− and TRIF−/− brain on day3 post-ICH. **P < 0.01 _vs_. sham group; ##_P_ < 0. 01 _vs_. WT group. P < 0.01 vs. WT group, Bar = 50 μM in D. Values (mean ± SD, n = 3 for each group) are representative of three independent experiments.
Figure 4
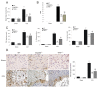
TLR4−/− mice showed reduced MyD88 and TRIF protein level and NF-κB activity. Western blot showed TLR4−/− had decreased expression of MyD88 (A) and TRIF (B). Electrophoretic mobility shift assay showed decreased NF-κB activity in TLR4−/− (C) **P < 0.01 vs. sham group, ##P < 0. 01 vs. WT group. Values (mean ± SD, n = 3 for each group) are representative of three independent experiments.
Figure 5
Heme stimulates microglia activation and induces brain inflammatory injury via TLR4. (A) Stimulation with bilirubin, Hemin and FeSO4 influenced TLR4 expression on cultured microglial cells. **P < 0.01 _vs_. control group (_n_ = 6) (**B**) TNF-α release in microglial cells in response to stimulus. **, ##_P_ < 0.01 _vs_. control group, P < 0.01 _vs_. LPS group (_n_ = 6). (**C**) TNF-α, IL-1β, and IL-6 expression in microglial cells in response to stimulus. **_P_ < 0.01 _vs_. control group, ##, P < 0.01 vs. WT group, n = 6. Hemin treatment decreased brain water content (D) and NDS (D) in TLR4−/− mice. **P < 0.01 vs. control group, ##P < 0.01 vs. WT group n = 6. (E) Hemin treatment increased cytokines release in TLR4−/− mice. **P < 0.01 vs. control group, ##P < 0.01 vs. WT group n = 6. Values (mean ± SD) are representative of two independent experiments.
Figure 6
Administration of anti-TLR4 monoclonal antibody immediately after ICH provided neuroprotection. Administration of antibody against TLR4 in WT mice of ICH decreased brain water content (A) and NDS (B). Administration of antibody against TLR4 in WT mice significantly decreased cytokine release (C) and macrophage infiltration (D) in response to ICH. **P < 0.01 vs. WT group. Scale bar = 50 μM (D). Values (mean ± SD, n = 6 for each group) are representative of three independent experiments.
Figure 7
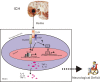
Schematic diagram of the Heme/TLR4/MyD88 and/or TRIF hypothesis in ICH. Heme activates TLR4-mediated inflammatory injury via the MyD88/TRIF signaling pathway in intracerebral hemorrhage mouse brain.
Similar articles
- TLR4-HMGB1-, MyD88- and TRIF-dependent signaling in mouse intestinal ischemia/reperfusion injury.
Wang J, He GZ, Wang YK, Zhu QK, Chen W, Guo T. Wang J, et al. World J Gastroenterol. 2015 Jul 21;21(27):8314-25. doi: 10.3748/wjg.v21.i27.8314. World J Gastroenterol. 2015. PMID: 26217083 Free PMC article. - Melatonin modulates TLR4-mediated inflammatory genes through MyD88- and TRIF-dependent signaling pathways in lipopolysaccharide-stimulated RAW264.7 cells.
Xia MZ, Liang YL, Wang H, Chen X, Huang YY, Zhang ZH, Chen YH, Zhang C, Zhao M, Xu DX, Song LH. Xia MZ, et al. J Pineal Res. 2012 Nov;53(4):325-34. doi: 10.1111/j.1600-079X.2012.01002.x. Epub 2012 Apr 27. J Pineal Res. 2012. PMID: 22537289 - Cold-inducible RNA-binding protein contributes to intracerebral hemorrhage-induced brain injury via TLR4 signaling.
Zhou K, Cui S, Duan W, Zhang J, Huang J, Wang L, Gong Z, Zhou Y. Zhou K, et al. Brain Behav. 2020 Jun;10(6):e01618. doi: 10.1002/brb3.1618. Epub 2020 Apr 13. Brain Behav. 2020. PMID: 32285591 Free PMC article. - The Pathologic Role of Toll-Like Receptor 4 in Prostate Cancer.
Ou T, Lilly M, Jiang W. Ou T, et al. Front Immunol. 2018 Jun 6;9:1188. doi: 10.3389/fimmu.2018.01188. eCollection 2018. Front Immunol. 2018. PMID: 29928275 Free PMC article. Review. - Role of Neutrophils as Therapeutic Targets in Intracerebral Hemorrhage.
Ardic AF, Ardic N. Ardic AF, et al. Ther Innov Regul Sci. 2024 Sep;58(5):807-816. doi: 10.1007/s43441-024-00668-9. Epub 2024 May 16. Ther Innov Regul Sci. 2024. PMID: 38753134 Review.
Cited by
- Protective effect of naringenin against experimental colitis via suppression of Toll-like receptor 4/NF-κB signalling.
Dou W, Zhang J, Sun A, Zhang E, Ding L, Mukherjee S, Wei X, Chou G, Wang ZT, Mani S. Dou W, et al. Br J Nutr. 2013 Aug;110(4):599-608. doi: 10.1017/S0007114512005594. Epub 2013 Mar 18. Br J Nutr. 2013. PMID: 23506745 Free PMC article. - Oxidative stress is related to the deleterious effects of heme oxygenase-1 in an in vivo neuroinflammatory rat model.
Tronel C, Rochefort GY, Arlicot N, Bodard S, Chalon S, Antier D. Tronel C, et al. Oxid Med Cell Longev. 2013;2013:264935. doi: 10.1155/2013/264935. Epub 2013 Mar 5. Oxid Med Cell Longev. 2013. PMID: 23533686 Free PMC article. - Role of heme in cardiovascular physiology and disease.
Sawicki KT, Chang HC, Ardehali H. Sawicki KT, et al. J Am Heart Assoc. 2015 Jan 5;4(1):e001138. doi: 10.1161/JAHA.114.001138. J Am Heart Assoc. 2015. PMID: 25559010 Free PMC article. Review. No abstract available. - Moderate Prenatal Alcohol Exposure Increases Toll-like Receptor Activity in Umbilical Cord Blood at Birth: A Pilot Study.
Maxwell JR, Noor S, Pavlik N, Rodriguez DE, Enriquez Marquez L, DiDomenico J, Blossom SJ, Bakhireva LN. Maxwell JR, et al. Int J Mol Sci. 2024 Jun 27;25(13):7019. doi: 10.3390/ijms25137019. Int J Mol Sci. 2024. PMID: 39000127 Free PMC article. - Regulatory T cells ameliorate intracerebral hemorrhage-induced inflammatory injury by modulating microglia/macrophage polarization through the IL-10/GSK3β/PTEN axis.
Zhou K, Zhong Q, Wang YC, Xiong XY, Meng ZY, Zhao T, Zhu WY, Liao MF, Wu LR, Yang YR, Liu J, Duan CM, Li J, Gong QW, Liu L, Yang MH, Xiong A, Wang J, Yang QW. Zhou K, et al. J Cereb Blood Flow Metab. 2017 Mar;37(3):967-979. doi: 10.1177/0271678X16648712. Epub 2016 Jul 20. J Cereb Blood Flow Metab. 2017. PMID: 27174997 Free PMC article. Retracted.
References
- Castillo J, Dávalos A, Alvarez-Sabín J, Pumar JM, Leira R, Silva Y, Montaner J, Kase CS. Molecular signatures of brain injury after intracerebral hemorrhage. Neurology. 2002;58:624–629. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources