Altered stability of mRNAs associated with glaucoma progression in human trabecular meshwork cells following oxidative stress - PubMed (original) (raw)
Altered stability of mRNAs associated with glaucoma progression in human trabecular meshwork cells following oxidative stress
Hideki Mochizuki et al. Invest Ophthalmol Vis Sci. 2012.
Abstract
Purpose: The goals of this study were to determine if oxidative stress on human trabecular meshwork (HTM) cells influences the stability of key mRNAs containing AU rich elements (AREs) known to be associated with glaucoma progression, and if the presence of topographic cue alters the stability of these mRNAs.
Methods: HTM cells were treated with 300 μM hydrogen peroxide (H(2)O(2)) for 1 hour in the presence of 5 μg/mL actinomycin D and compared with untreated cells. The selected mRNAs (IL-6, IL-8, myocilin, SPARC [secreted protein, acidic and rich in cysteine], matrix metalloproteinase [MMP]-3, and MMP-9) from the cells were analyzed by using relative quantitative PCR. Immunohistochemistry for Hu antigen R (HuR) was performed in addition to Western blots of HuR. HTM cells were also grown on topographically patterned surfaces, and IL-6 mRNA was analyzed by quantitative PCR.
Results: H(2)O(2) increased IL-6 mRNA stability 0.145 (0.095-0.27) to 0.345 (0.2-0.48) (normalized ratio, median [interquartile range]) (n = 5), while IL-8 mRNA was increased from 0.565 (0.408-0.6) to 0.775 (0.486-0.873) (n = 5). These differences were statistically significant (P = 0.0313, for both IL-6 and IL-8; Wilcoxon signed-rank test). The mRNAs of myocilin, SPARC, and MMP-3, which do not have AREs, were more stable after actinomycin D treatment and were not altered with oxidation. Western blot and immunohistochemistry demonstrated that H(2)O(2) treatment induces the translocation of HuR from the nucleus to the cytoplasm. Nanopatterned surfaces did not alter IL-6 mRNA stability.
Conclusions: Oxidative stress stabilizes IL-6 and IL-8 mRNAs significantly. The decay of certain mRNAs associated with glaucoma may be altered in the trabecular meshwork of glaucoma patients.
Figures
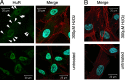
Figure 1.
Subcellular localization of HuR in HTM cells. (A) HuR (green) was visualized by laser confocal microscopy on cultured cells that were either left untreated or treated with 300 μM H2O2 (1 hour). DAPI (blue) staining served to visualize the nucleus and phalloidin (red) staining visualized actin in the cytoplasm. Note the distinct overlap of DAPI and HuR signals; while H2O2-treated cells also exhibited abundant nuclear HuR, the treatment caused an increase in the cytoplasmic HuR (arrow heads). (B) Negative control without primary antibody to HuR showed no reaction with the secondary antibody.
Figure 2.
Increased cytoplasmic HuR after exposure to oxidative stress. HTM cells were harvested 1 hour after incubation with or without 300 μM H2O2. Cytoplasmic and nuclear lysates were prepared, and HuR levels were determined by Western blotting. GAPDH and lamin B1 show definitive fractionation of the cytoplasm (C) and nucleus (N). GAPDH and lamin B1 were used as loading controls for cytoplasmic and nuclear samples, respectively. Cytoplasmic and nuclear HuR and were normalized for GAPDH or lamin B1 and the intensities of oxidatively stressed samples were compared with control samples. Cytoplasmic HuR was increased after exposure to H2O2 (average ratio 1.42, 95% confidential interval 1.04–1.81, P = 0.04, Student's _t_-test). In contrast, there was no statistical difference in nuclear HuR (n = 4, average ratio 1.26, 95% confidential interval 0.66–1.86, P = 0.26, Student's _t_-test).
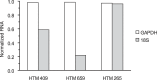
Figure 3.
Determination of endogenous control for mRNA stabilization. To select a proper internal control gene for real-time PCR analysis, we compared 18S rRNA to GAPDH mRNA expression in our experiments. Cell cultures from three different individuals were analyzed. HTM cells were treated with 5 μg/mL actinomycin D to suppress transcription (t = −30 minutes). After 30 minutes, cells were treated with 300 μM hydrogen peroxide (t = 0) and incubated for 1 hour (t = 1 hour). They were harvested at t = −30 minutes and t = 1 hour. The same amount of total cellular RNA was used to standardize the samples. Each real-time PCR amplification was run in triplicate. Data are shown as mean and are normalized to t = −30 minutes. GAPDH RNA appeared more consistent than 18S RNA. Therefore, we used GAPDH mRNA as an internal control in subsequent experiments.
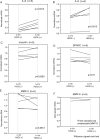
Figure 4.
Stability change of mRNA with H2O2 treatment in HTM cells. HTM cells were treated with 5 μg/mL actinomycin D to suppress transcription (t = −30 minutes). After 30 minutes, cells were treated with or without 300 μM H2O2 (t = 0) and incubated for 1 hour (t = 1 hour). They were harvested at t = −30min and t = 1 hour. Each real-time PCR amplification was run in triplicate. Data are shown as mean of the triplicate and normalized to t = −30 minutes. H2O2 increased IL-6 (A) and IL-8 (B) mRNA stability. The change of myocilin (C), SPARC (D), and MMP-3 (E) mRNA stability varied with each individual but was not significantly different between the treated and untreated cells. MMP-9 (F) did not vary much in the two samples.
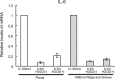
Figure 5.
Stability of mRNA for IL-6 with H2O2 treatment in HTM cells grown on 1400-nm pitch topography compared with planar surfaces. The result of one representative culture of two, obtained from a 26-year-old donor, is shown. HTM cells were treated with 5 μg/mL actinomycin D to suppress transcription (t = −30 minutes). Data are shown as mean ± standard deviation of the triplicate and normalized to t = −30 minutes of planar or topographic surfaces. No difference in stabilization of mRNA was noted between the cells on topography and planar substrates. Although nanopatterned surfaces influence a number of cellular behaviors, nanopatterned surfaces did not alter IL-6 mRNA stability. This result suggests topographic cues do not affect ARE stabilization by HuR.
Similar articles
- Substratum stiffness and latrunculin B regulate matrix gene and protein expression in human trabecular meshwork cells.
Thomasy SM, Wood JA, Kass PH, Murphy CJ, Russell P. Thomasy SM, et al. Invest Ophthalmol Vis Sci. 2012 Feb 23;53(2):952-8. doi: 10.1167/iovs.11-8526. Print 2012 Feb. Invest Ophthalmol Vis Sci. 2012. PMID: 22247475 Free PMC article. - NFATc1 activity regulates the expression of myocilin induced by dexamethasone.
Faralli JA, Clark RW, Filla MS, Peters DM. Faralli JA, et al. Exp Eye Res. 2015 Jan;130:9-16. doi: 10.1016/j.exer.2014.11.009. Epub 2014 Nov 18. Exp Eye Res. 2015. PMID: 25450062 Free PMC article. - Response of human trabecular meshwork cells to topographic cues on the nanoscale level.
Russell P, Gasiorowski JZ, Nealy PF, Murphy CJ. Russell P, et al. Invest Ophthalmol Vis Sci. 2008 Feb;49(2):629-35. doi: 10.1167/iovs.07-1192. Invest Ophthalmol Vis Sci. 2008. PMID: 18235008 Free PMC article. - The effects of myocilin expression on functionally relevant trabecular meshwork genes: a mini-review.
Borrás T. Borrás T. J Ocul Pharmacol Ther. 2014 Mar-Apr;30(2-3):202-12. doi: 10.1089/jop.2013.0218. Epub 2014 Feb 24. J Ocul Pharmacol Ther. 2014. PMID: 24564495 Free PMC article. Review. - Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma.
Wordinger RJ, Clark AF. Wordinger RJ, et al. Prog Retin Eye Res. 1999 Sep;18(5):629-67. doi: 10.1016/s1350-9462(98)00035-4. Prog Retin Eye Res. 1999. PMID: 10438153 Review.
Cited by
- Hydrogen peroxide sensing, signaling and regulation of transcription factors.
Marinho HS, Real C, Cyrne L, Soares H, Antunes F. Marinho HS, et al. Redox Biol. 2014 Feb 23;2:535-62. doi: 10.1016/j.redox.2014.02.006. eCollection 2014. Redox Biol. 2014. PMID: 24634836 Free PMC article. Review. - RNA-binding protein HuR enhances mineralocorticoid signaling in renal KC3AC1 cells under hypotonicity.
Lema I, Amazit L, Lamribet K, Fagart J, Blanchard A, Lombès M, Cherradi N, Viengchareun S. Lema I, et al. Cell Mol Life Sci. 2017 Dec;74(24):4587-4597. doi: 10.1007/s00018-017-2594-x. Epub 2017 Jul 25. Cell Mol Life Sci. 2017. PMID: 28744670 Free PMC article. - The Identification of New Pharmacological Targets for the Treatment of Glaucoma: A Network Pharmacology Approach.
Giuffrida E, Platania CBM, Lazzara F, Conti F, Marcantonio N, Drago F, Bucolo C. Giuffrida E, et al. Pharmaceuticals (Basel). 2024 Oct 5;17(10):1333. doi: 10.3390/ph17101333. Pharmaceuticals (Basel). 2024. PMID: 39458974 Free PMC article. - Central corneal thickness correlates with oxygen levels in the human anterior chamber angle.
Siegfried CJ, Shui YB, Bai F, Beebe DC. Siegfried CJ, et al. Am J Ophthalmol. 2015 Mar;159(3):457-62.e1. doi: 10.1016/j.ajo.2014.11.026. Epub 2014 Nov 26. Am J Ophthalmol. 2015. PMID: 25461296 Free PMC article. - NMI, POLR3G and APIP are the key molecules connecting glaucoma with high intraocular pressure: a clue for early diagnostic biomarker candidates.
Almarzouki N. Almarzouki N. Int J Ophthalmol. 2024 Nov 18;17(11):1987-1994. doi: 10.18240/ijo.2024.11.03. eCollection 2024. Int J Ophthalmol. 2024. PMID: 39559319 Free PMC article.
References
- Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol. 2004;137:62–69. - PubMed
- Beit-Yannai E, Trembovler V, Solomon AS. Decrease in reducing power of aqueous humor originating from glaucomatous rabbits. Eye. 2007;21:658–664. - PubMed
- Kahn MG, Giblin FJ, Epstein DL. Glutathione in calf trabecular meshwork and its relation to aqueous humor outflow facility. Invest Ophthalmol Vis Sci. 1983;24:1283–1287. - PubMed
- Sacca SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005;123:458–463. - PubMed
- Sacca SC, Izzotti A, Rossi P, Traverso C. Glaucomatous outflow pathway and oxidative stress. Exp Eye Res. 2007;84:389–399. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous