Restoration of synaptic plasticity and learning in young and aged NCAM-deficient mice by enhancing neurotransmission mediated by GluN2A-containing NMDA receptors - PubMed (original) (raw)
Comparative Study
Restoration of synaptic plasticity and learning in young and aged NCAM-deficient mice by enhancing neurotransmission mediated by GluN2A-containing NMDA receptors
Gaga Kochlamazashvili et al. J Neurosci. 2012.
Abstract
Neural cell adhesion molecule (NCAM) is the predominant carrier of the unusual glycan polysialic acid (PSA). Deficits in PSA and/or NCAM expression cause impairments in hippocampal long-term potentiation and depression (LTP and LTD) and are associated with schizophrenia and aging. In this study, we show that impaired LTP in adult NCAM-deficient (NCAM(-/-)) mice is restored by increasing the activity of the NMDA subtype of glutamate receptor (GluN) through either reducing the extracellular Mg2+ concentration or applying d-cycloserine (DCS), a partial agonist of the GluN glycine binding site. Pharmacological inhibition of the GluN2A subtype reduced LTP to the same level in NCAM(-/-) and wild-type (NCAM(+/+)) littermate mice and abolished the rescue by DCS in NCAM(-/-) mice, suggesting that the effects of DCS are mainly mediated by GluN2A. The insufficient contribution of GluN to LTD in NCAM(-/-) mice was also compensated for by DCS. Furthermore, impaired contextual and cued fear conditioning levels were restored in NCAM(-/-) mice by administration of DCS before conditioning. In 12-month-old NCAM(-/-), but not NCAM(+/+) mice, there was a decline in LTP compared with 3-month-old mice that could be rescued by DCS. In 24-month-old mice of both genotypes, there was a reduction in LTP that could be fully restored by DCS in NCAM(+/+) mice but only partially restored in NCAM(-/-) mice. Thus, several deficiencies of NCAM(-/-) mice can be ameliorated by enhancing GluN2A-mediated neurotransmission with DCS.
Figures
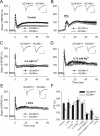
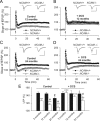
Figure 1.
NMDA receptor-dependent rather than
l
-type Ca2+ channel-dependent LTP is impaired in _NCAM_−/− mice. A, TBS-induced LTP is impaired in slices from _NCAM_−/− mice. B, Normal levels of
l
-type Ca2+ channel-dependent LTP are induced in _NCAM_−/− mice by TEA (25 m
m
, for 7 min, indicated by the horizontal bar) in the presence of the GluN antagonist APV. C, Increasing the extracellular Ca2+ concentration from 2.0 to 2.5 m
m
rescues LTP in _NCAM_−/− slices to the level of NCAM+/+ slices. D, Decreasing the extracellular Mg2+ concentration to 0.75 m
m
(instead of 1.5 m
m
) results in equal levels of LTP in the CA1 region of slices from _NCAM_−/− and NCAM+/+ mice. E, Restoration of impaired LTP in _NCAM_−/− mice by application of the GluN agonist DCS. A–E, Each value is an average of three consecutive time points. The mean slope of fEPSPs recorded 0–10 min before LTP induction is taken as 100%, and the arrows indicate the delivery of TBS. The data are presented as the mean + SEM. Each trace is the average of 30 fEPSPs recorded 10 min before or 50–60 min after LTP induction. Calibration: 1 mV and 20 ms. F, Means + SEMs of LTP levels recorded 50–60 min after LTP induction in the presence of GluN modulators. In addition to the effects depicted in panels (A–E), the graph shows that LTP is reduced to the same levels in both genotypes by the GluN2A antagonist NVP-AAM077, and it is fully blocked by the subtype-nonspecific GluN antagonist APV. Two-way ANOVA of all TBS-induced LTP values revealed effects of genotype (p = 0.023), treatment (p < 0.001), and a genotype × treatment interaction (p = 0.011). ***p < 0.001, significant difference between untreated NCAM+/+ and _NCAM_−/− slices; +++p ≤ 0.001, significant difference between untreated control and pharmacologically treated slices of the same genotype (SNK test). The numbers of tested NCAM+/+ and _NCAM_−/− slices/mice are, respectively: 24/19 and 20/17 (control), 13/6 and 12/5 (TEA), 8/5 and 8/4 (2.5 m
m
Ca2+), 9/6 and 8/4 (0.75 m
m
Mg2+), 11/4 and 12/5 (DCS), 10/8 and 7/6 (NVP-AAM077), 7/3 and 6/3 (NVP-AAM077 + DCS), and 6/3 and 7/3 (APV).
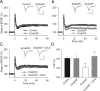
Figure 2.
Restoration of impaired LTP in endoNF treated wild-type hippocampal slices via facilitation of GluN receptors. A, LTP levels are normal in slices treated with the inactive form of endoNF (endoNF−) compared with sham-treated controls. B, In contrast, LTP is impaired in slices treated with the active form of endoNF. C, Restoration of LTP in endoNF treated slices to normal levels by application of the GluN agonist DCS. A–C, The presentation of traces and LTP profiles are as in Figure 1. Calibration: 1 mV and 20 ms. D, Means + SEMs of LTP levels recorded in wild-type slices 50–60 min after TBS application. One-way ANOVA revealed a significant difference between groups (p = 0.002). **p < 0.01, significant difference between endoNF treated slices and those either sham or endoNF− treated, ++p < 0.01, significant effects of DCS on endoNF-treated slices (SNK test). The number of tested slices/mice for each group is as follows: 8/8 (control), 6/5 (endoNF−), 9/9 (endoNF) and 8/5 (endoNF + DCS).
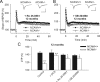
Figure 3.
Modulation of NMDA receptors restores impaired LTD in slices from _NCAM_−/− mice. A, Two trains of LFS (shown by the horizontal bar) induce LTD in untreated slices from NCAM+/+ mice but not _NCAM_−/− mice. B, Restoration of LTD in _NCAM_−/− mice and impairment of LTD in NCAM+/+ mice by application of the GluN agonist DCS. C, D, Inhibition of LTD in NCAM+/+ by the GluN2B selective antagonist Ro 25-6981 (C) and the preferentially GluN2A blocking NVP-AAM077 (D). A–D, Each value is an average of three consecutive time points. The mean slope of the fEPSPs recorded 0–10 min before LTD induction is taken as 100%. The data are presented as the ±SEM. Each trace is the average of 30 fEPSPs recorded 10 min before or 50–60 min after LTD induction. Calibration: 0.5 mV and 20 ms. E, Means + SEMs of LTD levels recorded 50–60 min after LTD induction in the presence of GluN modulators. Two-way ANOVA detected an effect of treatment (p = 0.02) and an interaction between treatment and genotype (p = 0.001). *p < 0.05 and ***p < 0.001, significant difference between NCAM+/+ and _NCAM_−/− mice treated with the same drug, +p < 0.05, ++p < 0.01, significant difference between control and pharmacologically treated mice of the same genotype (SNK test). The numbers of tested NCAM+/+ and _NCAM_−/− slices/mice for each group are as follows: 24/21 and 13/13 (control), 13/11 and 12/8 (DCS), 8/7 and 9/6 (Ro 25-6981) and 12/10 and 8/5 (NVP-AAM077).
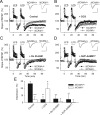
Figure 4.
Systemic administration of DCS restores contextual and tone memories in _NCAM_−/− mice. A, A schematic of the experiment; intraperitoneal injection of either drug or vehicle 15 min before the beginning of the training session; TR, training in the conditioned context CC+; B, baseline; d1 and d7, days 1 and 7 after training. B, A schematic of the retrieval tests at days 1 and 7; CC+ and CC−, conditioned and neutral contexts; CS+ and CS−, conditioned and neutral auditory stimuli. C, Levels of freezing before (baseline “B”, 180 s) and during the fear conditioning procedure. CS+: pairing of the conditioned tone (CS+, 20 s) with two footshocks (US), and PSI: poststimulus interval (60 s). Two-way repeated-measures ANOVA did not detect any differences between the six injected groups before or during training (p = 0.83). However, freezing increased significantly above the baseline after the first and second footshocks (p < 0.001). D, Contextual memory tests: levels of freezing on the two posttraining days d1 and d7 in the conditioned context (CC+, colorful bars) and in the control neutral context (CC−, white bars). The latter was assessed 3 min before tone presentation as shown in panel (B). All tests are taken from the same mice. E, Tone memory tests: levels of freezing during presentation of the conditioned tone (CS+, colorful bars) and the control tone (CS−, white bars) on the two posttraining days d1 and d7. The DCS dosages were 0 (vehicle, Veh), 3, 10, and 20 mg/kg of body weight; white color represents responses to the neutral context CC− and the auditory stimulus CS-. Two-way repeated-measures ANOVA revealed a difference in freezing in the CC+ between the six injected groups (p < 0.001). Freezing responses to both cued stimuli CS+ (p < 0.01) and CS− (p < 0.001) were also different between groups. +p < 0.05, ++p < 0.01, significant rescue by DCS (SNK test); **p < 0.01, ***p < 0.001, significant differences between genotypes (SNK test). #p < 0.05, significant differences between CC+ versus CC− or CS+ versus CS− (Wilcoxon test). The number of tested mice for each group: 11 (NCAM+/+, Veh), 8 (NCAM+/+, DCS 20 mg/kg), 7 (NCAM−/−, Veh), 7 (NCAM−/−, DCS 3 mg/kg), 8 (NCAM−/−, DCS 10 mg/kg), 8 (NCAM−/−, DCS 20 mg/kg).
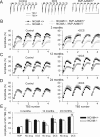
Figure 5.
Age-dependent decline of LTP in NCAM-deficient mice. A, Normal levels of LTP in slices from 12-month-old, as compared with 3-month-old, NCAM+/+ mice. In contrast, LTP is significantly diminished in slices from 12-month-old _NCAM_−/− mice. B, DCS effectively restores impaired LTP in slices from 12-month-old _NCAM_−/− mice. C, LTP levels are reduced in slices from 24-month-old NCAM+/+ mice and lower yet in NCAM−/− mice as compared with 12-month-old mutants. D, DCS effectively restores impaired LTP in slices from 24-month-old NCAM+/+ mice. Although LTP is significantly increased by DCS in slices from 24-month-old _NCAM_−/− mice, it is not restored to the level seen in NCAM+/+ controls. A–D, The presentation of traces and LTP profiles are as in Figure 1. Calibration: 1 mV and 20 ms. E, Means + SEMs of LTP levels recorded 50–60 min after TBS at different ages in control conditions and after treatment with the GluN agonist DCS. DCS effectively restores impaired LTP in slices from 24-month-old NCAM+/+ mice. Although LTP is significantly increased by DCS in slices from 24-month-old _NCAM_−/− mice, it is not restored to the level seen in NCAM+/+ controls. Three-way ANOVA revealed effects of genotype, age and treatment and an interaction between treatment and genotype (all p ≤ 0.001). *p < 0.05, ***p < 0.001, significant differences between NCAM+/+ and _NCAM_−/− mice treated in the same way; #p < 0.05, significant effect of aging in the same genotype (SNK test); +p ≤ 0.05, ++p < 0.01, +++p < 0.001, significant differences between untreated control and DCS-treated slices of the same genotype (t test). The numbers of tested NCAM+/+ and _NCAM_−/− slices/mice for each group are as follows: 24/19 and 20/17 (3-month-old, control), 11/4 and 12/5 (3-month-old, DCS), 9/3 and 9/3 (12-month-old, control), 9/4 and 7/4 (12-month-old, DCS), 8/4 and 6/3 (24-month-old, control), and 9/5 and 6/3 (24-month-old, DCS).
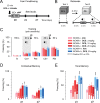
Figure 6.
Modulation of the late component of TBS-elicited fEPSPs by the GluN2A modulators DCS and NVP-AAM077. A, Examples of fEPSPs elicited by the first (black) and fourth (gray) TBS in 3-month-old NCAM+/+ mice, which were recorded in ACSF (Control) and after treatment with DCS or NVP-AAM077. Horizontal bars indicate the time intervals in which the mean amplitudes of the slow components following each theta-burst were measured. An increase in the amplitude of the slow components at the fourth TBS as compared with the first TBS is visible in controls and DCS-treated slices but not in NVP-AAM077-treated slices. Calibration: 1 mV and 500 ms. B–D, Modulation of the mean amplitude of slow fEPSP components during 4 TBSs in 3- (B), 12- (C), and 24-month-old (D) mice in control conditions (left) and after DCS application (right). A strong reduction of the slow component by NVP-AAM077 is illustrated in B. The data are presented as the ±SEM for fEPSPs collected during the LTP experiments shown in Figure 1 and 5. E, ±SEM values of slow fEPSPs elicited by the fourth TBS. *p < 0.05, **p < 0.01, significant differences between genotypes; +p < 0.05, ++p < 0.01, significant increases by DCS within a genotype (SNK test after two-way ANOVA for each age). The numbers of tested slices/mice for the NCAM+/+ and _NCAM_−/− genotypes are given in the legends to Figures 1 and 5.
Figure 7.
Partial restoration of impaired LTP in hippocampal slices from 12-month-old _NCAM_−/− mice through inhibition of GluN2B-containing receptor signaling. A, B, Partial rescue of impaired LTP in slices from 12-month-old _NCAM_−/− mice by application of the GluN2B selective antagonist Ro 25-6981 (A) or the p38 inhibitor SB 203580 (B). The presentation of traces and LTP profiles are as in Figure 1. Calibration: 1 mV and 20 ms. C, Means + SEMs of LTP levels recorded 50–60 min after TBS in control conditions and with different pharmacological treatments for 12-month-old mice. Two-way ANOVA revealed effects of genotype (p < 0.001) and a genotype × treatment interaction (p = 0.021). *p < 0.05, ***p < 0.001, significant differences between NCAM+/+ and _NCAM_−/− mice treated in the same way; +p < 0.05, ++p < 0.01, significant differences between untreated control and pharmacologically treated slices of the same genotype (SNK test). The numbers of tested 12-month-old NCAM+/+ and _NCAM_−/− slices/mice for each group are as follows: 9/3 and 9/3 (control), 9/4 and 7/4 (DCS), 6/3 and 8/4 (Ro 25-6981) and 7/4 and 8/4 (SB 203580).
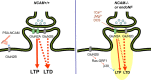
Figure 8.
Model for PSA-NCAM-mediated modulation of signaling via NMDA receptors. Endogenous and exogenous molecules are shown in black and brown, respectively. Stimulatory and inhibitory relationships are shown in red and blue, respectively. In NCAM+/+ mice, PSA-NCAM inhibits extrasynaptic GluN2B-containing receptors. LTP is induced through activation of GluN2A, whereas LTD requires the activity of both GluN2A- and GluN2B-containing receptors. In _NCAM_−/− mice, signal enhancement occurs via extrasynaptic GluN2B-containing receptors, whereas signal reduction occurs via GluN2A-containing receptors, which leads to impaired LTP and LTD. This model is supported by experiments from the present study with elevated extracellular Ca2+ and reduced extracellular Mg2+ or with DCS application and also by previous experiments applying Ro 25-6981, the glutamate scavenger GPT, PSA, or SB 203580 (Kochlamazashvili et al., 2010). All these treatments change the signaling balance between GluN2A- and GluN2B-mediated pathways in favor of the GluN2A-mediated pathway and restore LTP in _NCAM_−/− mice. Similarly, LTP is restored in endoNF treated slices from NCAM+/+ mice by DCS, Ro 25-6981, and SB 203580 and by genetic ablation of Ras-GRF1. Furthermore, fear memory is restored by DCS and Ro 25-6981 in _NCAM_−/− mice, whereas LTD is rescued by DCS.
Similar articles
- Neural cell adhesion molecule-associated polysialic acid regulates synaptic plasticity and learning by restraining the signaling through GluN2B-containing NMDA receptors.
Kochlamazashvili G, Senkov O, Grebenyuk S, Robinson C, Xiao MF, Stummeyer K, Gerardy-Schahn R, Engel AK, Feig L, Semyanov A, Suppiramaniam V, Schachner M, Dityatev A. Kochlamazashvili G, et al. J Neurosci. 2010 Mar 17;30(11):4171-83. doi: 10.1523/JNEUROSCI.5806-09.2010. J Neurosci. 2010. PMID: 20237287 Free PMC article. - Mice deficient in the polysialyltransferase ST8SiaIV/PST-1 allow discrimination of the roles of neural cell adhesion molecule protein and polysialic acid in neural development and synaptic plasticity.
Eckhardt M, Bukalo O, Chazal G, Wang L, Goridis C, Schachner M, Gerardy-Schahn R, Cremer H, Dityatev A. Eckhardt M, et al. J Neurosci. 2000 Jul 15;20(14):5234-44. doi: 10.1523/JNEUROSCI.20-14-05234.2000. J Neurosci. 2000. PMID: 10884307 Free PMC article. - PSA-NCAM is required for activity-induced synaptic plasticity.
Muller D, Wang C, Skibo G, Toni N, Cremer H, Calaora V, Rougon G, Kiss JZ. Muller D, et al. Neuron. 1996 Sep;17(3):413-22. doi: 10.1016/s0896-6273(00)80174-9. Neuron. 1996. PMID: 8816705 - The neural cell adhesion molecule in synaptic plasticity and ageing.
Rønn LC, Berezin V, Bock E. Rønn LC, et al. Int J Dev Neurosci. 2000 Apr-Jun;18(2-3):193-9. doi: 10.1016/s0736-5748(99)00088-x. Int J Dev Neurosci. 2000. PMID: 10715574 Review. - Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors.
MacDonald JF, Jackson MF, Beazely MA. MacDonald JF, et al. Crit Rev Neurobiol. 2006;18(1-2):71-84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. Crit Rev Neurobiol. 2006. PMID: 17725510 Review.
Cited by
- Synaptic cell adhesion molecules contribute to the pathogenesis and progression of fragile X syndrome.
Bai SY, Zeng DY, Ouyang M, Zeng Y, Tan W, Xu L. Bai SY, et al. Front Cell Neurosci. 2024 Jul 3;18:1393536. doi: 10.3389/fncel.2024.1393536. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39022311 Free PMC article. Review. - Depletion of polysialic acid from neural cell adhesion molecule (PSA-NCAM) increases CA3 dendritic arborization and increases vulnerability to excitotoxicity.
McCall T, Weil ZM, Nacher J, Bloss EB, El Maarouf A, Rutishauser U, McEwen BS. McCall T, et al. Exp Neurol. 2013 Mar;241:5-12. doi: 10.1016/j.expneurol.2012.11.028. Epub 2012 Dec 7. Exp Neurol. 2013. PMID: 23219884 Free PMC article. - Environmental Enrichment Ameliorates Behavioral Impairments Modeling Schizophrenia in Mice Lacking Metabotropic Glutamate Receptor 5.
Burrows EL, McOmish CE, Buret LS, Van den Buuse M, Hannan AJ. Burrows EL, et al. Neuropsychopharmacology. 2015 Jul;40(8):1947-56. doi: 10.1038/npp.2015.44. Epub 2015 Feb 10. Neuropsychopharmacology. 2015. PMID: 25666312 Free PMC article. - Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration.
Schnaar RL, Gerardy-Schahn R, Hildebrandt H. Schnaar RL, et al. Physiol Rev. 2014 Apr;94(2):461-518. doi: 10.1152/physrev.00033.2013. Physiol Rev. 2014. PMID: 24692354 Free PMC article. Review. - Sialometabolism in Brain Health and Alzheimer's Disease.
Rawal P, Zhao L. Rawal P, et al. Front Neurosci. 2021 Mar 30;15:648617. doi: 10.3389/fnins.2021.648617. eCollection 2021. Front Neurosci. 2021. PMID: 33867926 Free PMC article. Review.
References
- Abrous DN, Montaron MF, Petry KG, Rougon G, Darnaudéry M, Le Moal M, Mayo W. Decrease in highly polysialylated neuronal cell adhesion molecules and in spatial learning during ageing are not correlated. Brain Res. 1997;744:285–292. - PubMed
- Aniksztejn L, Ben-Ari Y. Novel form of long-term potentiation produced by a K+ channel blocker in the hippocampus. Nature. 1991;349:67–69. - PubMed
- Aura J, Riekkinen P., Jr Pre-training blocks the improving effect of tetrahydroaminoacridine and d-cycloserine on spatial navigation performance in aged rats. Eur J Pharmacol. 2000;390:313–318. - PubMed
- Aura J, Riekkinen M, Riekkinen P., Jr Tetrahydroaminoacridine and d-cycloserine stimulate acquisition of water maze spatial navigation in aged rats. Eur J Pharmacol. 1998;342:15–20. - PubMed
- Bado P, Madeira C, Vargas-Lopes C, Moulin TC, Wasilewska-Sampaio AP, Maretti L, de Oliveira RV, Amaral OB, Panizzutti R. Effects of low-dose D-serine on recognition and working memory in mice. Psychopharmacology. 2011;218:461–470. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous