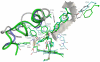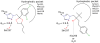Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus - PubMed (original) (raw)
Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus
Eveline Snelders et al. PLoS One. 2012.
Abstract
Background: Azoles play an important role in the management of Aspergillus diseases. Azole resistance is an emerging global problem in Aspergillus fumigatus, and may develop through patient therapy. In addition, an environmental route of resistance development has been suggested through exposure to 14α-demethylase inhibitors (DMIs). The main resistance mechanism associated with this putative fungicide-driven route is a combination of alterations in the Cyp51A-gene (TR(34)/L98H). We investigated if TR(34)/L98H could have developed through exposure to DMIs.
Methods and findings: Thirty-one compounds that have been authorized for use as fungicides, herbicides, herbicide safeners and plant growth regulators in The Netherlands between 1970 and 2005, were investigated for cross-resistance to medical triazoles. Furthermore, CYP51-protein homology modeling and molecule alignment studies were performed to identify similarity in molecule structure and docking modes. Five triazole DMIs, propiconazole, bromuconazole, tebuconazole, epoxiconazole and difenoconazole, showed very similar molecule structures to the medical triazoles and adopted similar poses while docking the protein. These DMIs also showed the greatest cross-resistance and, importantly, were authorized for use between 1990 and 1996, directly preceding the recovery of the first clinical TR(34)/L98H isolate in 1998. Through microsatellite genotyping of TR(34)/L98H isolates we were able to calculate that the first isolate would have arisen in 1997, confirming the results of the abovementioned experiments. Finally, we performed induction experiments to investigate if TR(34)/L98H could be induced under laboratory conditions. One isolate evolved from two copies of the tandem repeat to three, indicating that fungicide pressure can indeed result in these genomic changes.
Conclusions: Our findings support a fungicide-driven route of TR(34)/L98H development in A. fumigatus. Similar molecule structure characteristics of five triazole DMIs and the three medical triazoles appear the underlying mechanism of cross resistance development. Our findings have major implications for the assessment of health risks associated with the use of triazole DMIs.
Conflict of interest statement
Competing Interests: Research grants from Pfizer, Merck, Gilead Sciences, Cephalon, Astellas, and Basilea. These research grants were not awarded for the research described in this manuscript. The other authors have declared that no competing interests exist. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Figures
Figure 1. Chemical structures of antifungal compounds.
Three medical antifungal compounds and 31 compounds that were authorized by the Dutch Board for the Authorization of Plant Protection Products and Biocides for use as fungicides, herbicides, herbicide safeners and plant growth regulators. The compounds are presented according to structural group.
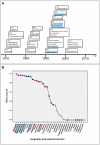
Figure 2. Overview of introduction of the 31 compounds by year and correlation effect sizes.
A) Overview of compounds by year of authorization by the Dutch Board for the Authorization of Plant Protection Products and Biocides (data from the Dutch Foundation for Phytofarmacy, Nefyto). The five triazole DMIs that exhibited the most identical docking by molecule alignment are underlined in blue. B) Correlation effect sizes (r) of compounds and medical triazoles comparing differences in the median MIC of wild type and TR34/L98H isolates. The fungicides are represented by grey dots and those belonging to the DMIs by black. The medical triazoles are indicated in red, and the five triazole DMIs that exhibited the most identical docking by molecule alignment are indicated in blue. *Correlation effect sizes could not be computed if in at least one of the two groups all variables were constant. This was the case with compounds that showed no in vitro activity against both wild type and TR34/L98H A. fumigatus isolates, and the correlation effect size was considered 0.
Figure 3. 3D representation of three aligned structures of CYP51 with the ligands in their active site, constructed by using the Yasara software.
In green human CYP51 bound with ketoconazole from PDB: 3I3K; in gray Mt bound with fluconazole from PDB: 1EA1; in cyan A. fumigatus bound with ketoconazole from the homology model. The ligands are represented in balls and sticks, only the residues important for binding a particular ligand are depicted in the picture and represented in sticks. Numbering of the residues corresponds with their colors to the models.
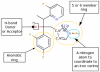
Figure 4. Two-dimensional structure of voriconazole with indicated pharmacophores that were used to align and filter the 31 compounds ( Table 1 ).
The figure was constructed by using Marvin Sketcher form ChemAxon (
).
Figure 5. Analysis of most modes binding modes compared to the medical triazoles.
A) Binding modes of propiconazole. This fungicide exhibits the most similar binding modes compared to the medical triazoles located in the active site of human and A. fumigatus CYP51. B) Binding modes of tebuconazole. This fungicide exhibits the most similar binding modes compared to the medical triazoles located in the active site of Mt CYP51. The main difference between A and B is the interactions with residue H296 in the active site, which is lacking in A.
Figure 6. The evolution of new microsatellite genotypes over time based on short tandem repeat typing of 144 TR34/L98H A. fumigatus isolates, cultured between 1998 and 2009 in the Netherlands.
By plotting the number of observed new genotypes versus time on a semi-logarithmic scale, a rate of change of 1.37±0.05 genotype-1.year-1 was calculated. As the first TR34/L98H isolate was cultured in 1998, the rate of change indicates that the first strain would have emerged around 1997 (95% CI: 1993.7–1999.7). This analysis also indicates that TR34/L98H had developed from a single ancestor.
Similar articles
- Environmental fungicides and triazole resistance in Aspergillus.
Bowyer P, Denning DW. Bowyer P, et al. Pest Manag Sci. 2014 Feb;70(2):173-8. doi: 10.1002/ps.3567. Epub 2013 Dec 5. Pest Manag Sci. 2014. PMID: 23616354 - Clonal expansion and emergence of environmental multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR₃₄/L98H mutations in the cyp51A gene in India.
Chowdhary A, Kathuria S, Xu J, Sharma C, Sundar G, Singh PK, Gaur SN, Hagen F, Klaassen CH, Meis JF. Chowdhary A, et al. PLoS One. 2012;7(12):e52871. doi: 10.1371/journal.pone.0052871. Epub 2012 Dec 28. PLoS One. 2012. PMID: 23285210 Free PMC article. - Isolation of multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR/L98H mutations in the cyp51A gene in India.
Chowdhary A, Kathuria S, Randhawa HS, Gaur SN, Klaassen CH, Meis JF. Chowdhary A, et al. J Antimicrob Chemother. 2012 Feb;67(2):362-6. doi: 10.1093/jac/dkr443. Epub 2011 Oct 25. J Antimicrob Chemother. 2012. PMID: 22028200 - Azole resistant Aspergillus fumigatus: an emerging problem.
Lelièvre L, Groh M, Angebault C, Maherault AC, Didier E, Bougnoux ME. Lelièvre L, et al. Med Mal Infect. 2013 Apr;43(4):139-45. doi: 10.1016/j.medmal.2013.02.010. Epub 2013 Apr 4. Med Mal Infect. 2013. PMID: 23562488 Review. - [Azole resistance in Aspergillus fumigatus in the Netherlands--increase due to environmental fungicides?].
Verweij PE, van de Sande-Bruisma N, Kema GH, Melchers WJ. Verweij PE, et al. Ned Tijdschr Geneeskd. 2012;156(25):A4458. Ned Tijdschr Geneeskd. 2012. PMID: 22748367 Review. Dutch.
Cited by
- Understanding the clinical and environmental drivers of antifungal resistance in the One Health context.
Williams CC, Gregory JB, Usher J. Williams CC, et al. Microbiology (Reading). 2024 Oct;170(10):001512. doi: 10.1099/mic.0.001512. Microbiology (Reading). 2024. PMID: 39475703 Free PMC article. Review. - Is the emergence of fungal resistance to medical triazoles related to their use in the agroecosystems? A mini review.
Ribas E Ribas AD, Spolti P, Del Ponte EM, Donato KZ, Schrekker H, Fuentefria AM. Ribas E Ribas AD, et al. Braz J Microbiol. 2016 Oct-Dec;47(4):793-799. doi: 10.1016/j.bjm.2016.06.006. Epub 2016 Jul 7. Braz J Microbiol. 2016. PMID: 27544394 Free PMC article. Review. - Gene expression, molecular docking, and molecular dynamics studies to identify potential antifungal compounds targeting virulence proteins/genes VelB and THR as possible drug targets against Curvularia lunata.
Kamboj H, Gupta L, Kumar P, Sen P, Sengupta A, Vijayaraghavan P. Kamboj H, et al. Front Mol Biosci. 2022 Dec 12;9:1055945. doi: 10.3389/fmolb.2022.1055945. eCollection 2022. Front Mol Biosci. 2022. PMID: 36619165 Free PMC article. - Triazole-Resistant Aspergillus fumigatus from Fungicide-Experienced Soils in Naivasha Subcounty and Nairobi County, Kenya.
Kemoi EK, Nyerere A, Bii CC. Kemoi EK, et al. Int J Microbiol. 2018 Jun 26;2018:7147938. doi: 10.1155/2018/7147938. eCollection 2018. Int J Microbiol. 2018. PMID: 30046310 Free PMC article. - Molecular mechanisms of acquired antifungal drug resistance in principal fungal pathogens and EUCAST guidance for their laboratory detection and clinical implications.
Rogers TR, Verweij PE, Castanheira M, Dannaoui E, White PL, Arendrup MC; Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). Rogers TR, et al. J Antimicrob Chemother. 2022 Jul 28;77(8):2053-2073. doi: 10.1093/jac/dkac161. J Antimicrob Chemother. 2022. PMID: 35703391 Free PMC article. Review.
References
- Pascual A, Calandra T, Bolay S, Buclin T, Bille J, et al. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008;46:201–211. - PubMed
- Kuipers S, Bruggemann RJ, de Sevaux RG, Heesakkers JP, Melchers WJ, et al. Failure of posaconazole therapy in a renal transplant patient with invasive aspergillosis due to Aspergillus fumigatus with attenuated susceptibility to posaconazole. Antimicrob Agents Chemother. 2011;55:3564–3566. - PMC - PubMed
- Verweij PE, Mellado E, Melchers W. Multiple-triazole-resistant aspergillosis. N Engl J Med. 2007;356:1481–1483. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources