Functional STAT3 deficiency compromises the generation of human T follicular helper cells - PubMed (original) (raw)
. 2012 Apr 26;119(17):3997-4008.
doi: 10.1182/blood-2011-11-392985. Epub 2012 Mar 8.
Danielle T Avery, Anna Chan, Marcel Batten, Jacinta Bustamante, Stephanie Boisson-Dupuis, Peter D Arkwright, Alexandra Y Kreins, Diana Averbuch, Dan Engelhard, Klaus Magdorf, Sara S Kilic, Yoshiyuki Minegishi, Shigeaki Nonoyama, Martyn A French, Sharon Choo, Joanne M Smart, Jane Peake, Melanie Wong, Paul Gray, Matthew C Cook, David A Fulcher, Jean-Laurent Casanova, Elissa K Deenick, Stuart G Tangye
Affiliations
- PMID: 22403255
- PMCID: PMC3355712
- DOI: 10.1182/blood-2011-11-392985
Functional STAT3 deficiency compromises the generation of human T follicular helper cells
Cindy S Ma et al. Blood. 2012.
Abstract
T follicular helper (Tfh) cells are critical for providing the necessary signals to induce differentiation of B cells into memory and Ab-secreting cells. Accordingly, it is important to identify the molecular requirements for Tfh cell development and function. We previously found that IL-12 mediates the differentiation of human CD4(+) T cells to the Tfh lineage, because IL-12 induces naive human CD4(+) T cells to acquire expression of IL-21, BCL6, ICOS, and CXCR5, which typify Tfh cells. We have now examined CD4(+) T cells from patients deficient in IL-12Rβ1, TYK2, STAT1, and STAT3 to further explore the pathways involved in human Tfh cell differentiation. Although STAT1 was dispensable, mutations in IL12RB1, TYK2, or STAT3 compromised IL-12-induced expression of IL-21 by human CD4(+) T cells. Defective expression of IL-21 by STAT3-deficient CD4(+) T cells resulted in diminished B-cell helper activity in vitro. Importantly, mutations in STAT3, but not IL12RB1 or TYK2, also reduced Tfh cell generation in vivo, evidenced by decreased circulating CD4(+)CXCR5(+) T cells. These results highlight the nonredundant role of STAT3 in human Tfh cell differentiation and suggest that defective Tfh cell development and/or function contributes to the humoral defects observed in STAT3-deficient patients.
Figures
Figure 1
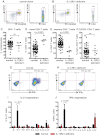
Naive CD4+ T cells deficient for IL-12Rβ1 fail to differentiate into IL-21–expressing cells in response to IL-12. (A-F) The frequency of total CD4+ T cells, and naive (CD45RA+CCR7+), memory (CD45RA+CCR7−/+), and CXCR5+CD45RA− CD4+ T cells, in PBMCs was determined for healthy donors and patients deficient for IL-12Rβ1. (A-B) Representative dot plots from 1 donor and 1 patient. (C-F) The frequency of (C) total, (D) naive (CD45RA+CCR7+), (E) memory (CD45RA+CCR7−/+), and (F) CXCR5+CD45RA− CD4+ T cells from all healthy donors (total CD4+ T cells, n = 54; naive CD4+ T cells, n = 70; memory CD4+ T cells, n = 70; CXCR5+CD45RA− CD4+ T cells, n = 61) and patients deficient for IL-12Rβ1 examined (n = 6). (G-J) Naive CD4+ T cells isolated from healthy donors (n = 5) and patients deficient for IL-12Rβ1 (n = 5) were cultured for 5 days under neutral (nil); polarizing Th1, Th2, or Th17 conditions; or in the presence of IL-6, IL-21, IL-23, or IL-27, and intracellular expression of IL-21 and IFNγ were then determined. (G-H) Representative dot plots of IL-21 and IFNγ expression by activated naive CD4+ T cells from 1 donor and 1 patient deficient for IL-12Rβ1. (I-J) Percentage of activated normal and IL-12Rβ1–deficient naive CD4+ T cells induced to express (I) IL-21 or (J) IFNγ in response to the indicated culture. The values represent the mean ± SEM.
Figure 2
STAT1 is dispensable for IL-12–induced expression of IL-21 in human CD4+ T cells. (A-E) The frequency of naive (CD45RA+CCR7+), memory (CD45RA+CCR7−/+), and CXCR5+CD45RA− CD4+ T cells in PBMCs was determined for healthy donors and STAT1-deficient patients. (A-B) Representative dot plots from 1 donor and 1 STAT1-deficient patient. (C-E) The frequency of (C) naive (CD45RA+CCR7+), (D) memory (CD45RA+CCR7−/+), and (E) CXCR5+CD45RA− CD4+ T cells from all healthy donors (total CD4+ T cells, n = 54; naive CD4+ T cells, n = 70; memory CD4+ T cells, n = 70; CXCR5+CD45RA− CD4+ T cells, n = 61) and STAT1-deficient patients (n = 6) was examined. (F-I) Total CD4+ T cells isolated from healthy donors and STAT1-deficient patients (n = 3) were cultured for 5 days under neutral (nil) or Th1-polarizing (ie, IL-12) conditions, and expression of intracellular IL-21 (F-G) and IFNγ (H-I) was then determined. The graphs in panels F and H show the frequency of cytokine-positive cells; those in panels G and I depict cytokine expression after Th1 polarization as fold-increase relative to the nil culture in each experiment. The values represent the mean ± SEM (n = 3).
Figure 3
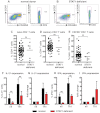
Mutations in STAT3 compromise the generation of CD4+ memory and CXCR5+CD45RA− Tfh cells. (A-B) The frequency of naive (CD45RA+CCR7+), memory (CD45RA+CCR7−/+), and CXCR5+CD45RA− CD4+ T cells in PBMCs was determined for healthy donors and STAT3MUT patients. (A-B) Representative dot plots from 1 donor and 1 patient. (C-F) The frequency of (C) total CD4+ T cells, (D) naive (CD45RA+CCR7+), (E) memory (CD45RA+CCR7−/+), and (F) CXCR5+CD45RA− CD4+ T cells from all healthy donors (total CD4+ T cells, n = 54; naive CD4+ T cells, n = 70; memory CD4+ T cells, n = 70; CXCR5+CD45RA− CD4+ T cells, n = 61) and STAT3MUT patients (n = 14) was examined.
Figure 4
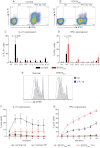
STAT3 mutations impair IL-12–induced expression of IL-21 in naive CD4+ T cells. (A-D) Naive CD4+ T cells isolated from healthy donors (n = 8) and STAT3MUT patients (n = 9) were cultured for 5 days under neutral conditions (nil); polarizing Th1, Th2, or Th17 conditions; or in the presence of IL-6, IL-21, IL-23, or IL-27, after which time expression of intracellular IL-21 and IFNγ was determined. (A-B) Representative dot plots of cytokine expression by activated naive CD4+ T cells from 1 donor and 1 patient. (C-D) Percentage of activated normal and STAT3MUT naive CD4+ T cells induced to express (C) IL-21 or (D) IFNγ in response to the indicated culture. The values represent the mean ± SEM. (E-F) Naive CD4+ T cells isolated from healthy donors and STAT3MUT patients were labeled with CFSE and cultured with anti-CD2/CD3/CD28 Abs in the absence (nil) or presence of Th1 polarizing conditions (+IL-12). After 5 days cells were harvested, and (E) proliferation and expression of (F) IL-21 and (G) IFNγ in cells that had undergone different divisions were then determined. (F-G) The values represent the mean ± SEM (n = 3).
Figure 5
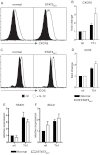
Induction of CXCR5, ICOS, and BCL6 by IL-12 in CD4+ T cells is independent of STAT3. (A-D) Naive CD4+ T cells isolated from healthy donors and STAT3MUT patients were cultured with anti-CD2/CD3/CD28 Abs in the absence (nil) or presence of Th1-polarizing conditions (+IL-12). After 4 days the cells were harvested, and surface expression of CXCR5 and ICOS was determined by flow cytometry and of TBX21 and BCL6 by qPCR. (A,C) representative histogram plots from 1 healthy donor and 1 STAT3MUT patient. Expression of (B) CXCR5 (n = 3), (D) ICOS (n = 3), (E) TBX21 (n = 4), and (F) BCL6 (n = 5) after Th1 polarization is presented as fold-increase compared with the nil culture in each experiment. (B,D-F) The graphs represent the mean ± SEM of the indicated number of experiments.
Figure 6
STAT3-deficient cells show impaired Tfh cell function in vitro. Naive CD4+ T cells isolated from healthy donors (HD) and STAT3MUT patients (Pt) were cultured under neutral (nil) or Th1 polarizing conditions. After 5 days, the cells were harvested and treated with mitomycin C before being cocultured with allogeneic naive B cells, in the absence or presence of exogenous IL-21, for an additional 7 days. After this time secretion of IgM, IgG, and IgA was determined. (A-B) The data were derived from experiments that used naive CD4+ T cells isolated from different STAT3MUT patients; (C) the data show the effect of exogenous IL-21 on the ability of STAT3MUT CD4+ T cells to provide B-cell help. Each graph represents the mean ± SEM of triplicate cultures; similar results were obtained in 5 (A-B) and 2 (C) experiments.
Similar articles
- Monogenic mutations differentially affect the quantity and quality of T follicular helper cells in patients with human primary immunodeficiencies.
Ma CS, Wong N, Rao G, Avery DT, Torpy J, Hambridge T, Bustamante J, Okada S, Stoddard JL, Deenick EK, Pelham SJ, Payne K, Boisson-Dupuis S, Puel A, Kobayashi M, Arkwright PD, Kilic SS, El Baghdadi J, Nonoyama S, Minegishi Y, Mahdaviani SA, Mansouri D, Bousfiha A, Blincoe AK, French MA, Hsu P, Campbell DE, Stormon MO, Wong M, Adelstein S, Smart JM, Fulcher DA, Cook MC, Phan TG, Stepensky P, Boztug K, Kansu A, İkincioğullari A, Baumann U, Beier R, Roscioli T, Ziegler JB, Gray P, Picard C, Grimbacher B, Warnatz K, Holland SM, Casanova JL, Uzel G, Tangye SG. Ma CS, et al. J Allergy Clin Immunol. 2015 Oct;136(4):993-1006.e1. doi: 10.1016/j.jaci.2015.05.036. Epub 2015 Jul 7. J Allergy Clin Immunol. 2015. PMID: 26162572 Free PMC article. - Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells.
Ray JP, Marshall HD, Laidlaw BJ, Staron MM, Kaech SM, Craft J. Ray JP, et al. Immunity. 2014 Mar 20;40(3):367-77. doi: 10.1016/j.immuni.2014.02.005. Epub 2014 Mar 13. Immunity. 2014. PMID: 24631156 Free PMC article. - Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation.
Choi YS, Eto D, Yang JA, Lao C, Crotty S. Choi YS, et al. J Immunol. 2013 Apr 1;190(7):3049-53. doi: 10.4049/jimmunol.1203032. Epub 2013 Feb 27. J Immunol. 2013. PMID: 23447690 Free PMC article. - Role of TRAFs in Signaling Pathways Controlling T Follicular Helper Cell Differentiation and T Cell-Dependent Antibody Responses.
Pedros C, Altman A, Kong KF. Pedros C, et al. Front Immunol. 2018 Oct 22;9:2412. doi: 10.3389/fimmu.2018.02412. eCollection 2018. Front Immunol. 2018. PMID: 30405612 Free PMC article. Review. - Transcriptional and epigenetic regulation of follicular T-helper cells and their role in autoimmunity.
Qiu H, Wu H, Chan V, Lau CS, Lu Q. Qiu H, et al. Autoimmunity. 2017 Mar;50(2):71-81. doi: 10.1080/08916934.2017.1284821. Epub 2017 Feb 21. Autoimmunity. 2017. PMID: 28263097 Review.
Cited by
- Dedicator of cytokinesis 8-deficient CD4+ T cells are biased to a TH2 effector fate at the expense of TH1 and TH17 cells.
Tangye SG, Pillay B, Randall KL, Avery DT, Phan TG, Gray P, Ziegler JB, Smart JM, Peake J, Arkwright PD, Hambleton S, Orange J, Goodnow CC, Uzel G, Casanova JL, Lugo Reyes SO, Freeman AF, Su HC, Ma CS. Tangye SG, et al. J Allergy Clin Immunol. 2017 Mar;139(3):933-949. doi: 10.1016/j.jaci.2016.07.016. Epub 2016 Aug 20. J Allergy Clin Immunol. 2017. PMID: 27554822 Free PMC article. - The cup runneth over: lessons from the ever-expanding pool of primary immunodeficiency diseases.
Milner JD, Holland SM. Milner JD, et al. Nat Rev Immunol. 2013 Sep;13(9):635-48. doi: 10.1038/nri3493. Epub 2013 Jul 26. Nat Rev Immunol. 2013. PMID: 23887241 Review. - Unique and shared signaling pathways cooperate to regulate the differentiation of human CD4+ T cells into distinct effector subsets.
Ma CS, Wong N, Rao G, Nguyen A, Avery DT, Payne K, Torpy J, O'Young P, Deenick E, Bustamante J, Puel A, Okada S, Kobayashi M, Martinez-Barricarte R, Elliott M, Sebnem Kilic S, El Baghdadi J, Minegishi Y, Bousfiha A, Robertson N, Hambleton S, Arkwright PD, French M, Blincoe AK, Hsu P, Campbell DE, Stormon MO, Wong M, Adelstein S, Fulcher DA, Cook MC, Stepensky P, Boztug K, Beier R, Ikincioğullari A, Ziegler JB, Gray P, Picard C, Boisson-Dupuis S, Phan TG, Grimbacher B, Warnatz K, Holland SM, Uzel G, Casanova JL, Tangye SG. Ma CS, et al. J Exp Med. 2016 Jul 25;213(8):1589-608. doi: 10.1084/jem.20151467. Epub 2016 Jul 11. J Exp Med. 2016. PMID: 27401342 Free PMC article. - STAT3, a Master Regulator of Anti-Tumor Immune Response.
Rébé C, Ghiringhelli F. Rébé C, et al. Cancers (Basel). 2019 Aug 30;11(9):1280. doi: 10.3390/cancers11091280. Cancers (Basel). 2019. PMID: 31480382 Free PMC article. Review. - Revisiting the Concept of Targeting NFAT to Control T Cell Immunity and Autoimmune Diseases.
Lee JU, Kim LK, Choi JM. Lee JU, et al. Front Immunol. 2018 Nov 27;9:2747. doi: 10.3389/fimmu.2018.02747. eCollection 2018. Front Immunol. 2018. PMID: 30538703 Free PMC article. Review.
References
- Tangye SG, Deenick EK, Palendira U, Ma CS. T cell-B cell interactions in primary immunodeficiencies. Ann N Y Acad Sci. 2012;1250(1):1–13. - PubMed
- Chtanova T, Tangye SG, Newton R, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173(1):68–78. - PubMed
- Yu D, Rao S, Tsai LM, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31(3):457–468. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous