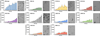Semisynthetic, site-specific ubiquitin modification of α-synuclein reveals differential effects on aggregation - PubMed (original) (raw)
. 2012 Mar 28;134(12):5468-71.
doi: 10.1021/ja300094r. Epub 2012 Mar 14.
Affiliations
- PMID: 22404520
- PMCID: PMC3315850
- DOI: 10.1021/ja300094r
Semisynthetic, site-specific ubiquitin modification of α-synuclein reveals differential effects on aggregation
Franziska Meier et al. J Am Chem Soc. 2012.
Abstract
The process of neurodegeneration in Parkinson's Disease is intimately associated with the aggregation of the protein α-synuclein into toxic oligomers and fibrils. Interestingly, many of these protein aggregates are found to be post-translationally modified by ubiquitin at several different lysine residues. However, the inability to generate homogeneously ubiquitin modified α-synuclein at each site has prevented the understanding of the specific biochemical consequences. We have used protein semisynthesis to generate nine site-specifically ubiquitin modified α-synuclein derivatives and have demonstrated that different ubiquitination sites have differential effects on α-synuclein aggregation.
Figures
Figure 1
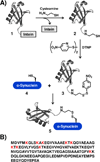
Disulfide-directed ubiquitination of α-synuclein. A) Ubiquitin was expressed in E. coli as a fusion to the GyrA intein (1). Intein-mediated thiolysis with cysteamine yielded 2 and subsequent reaction with DTNP generated the activated ubiquitin mixed disulfide (3). α-Synuclein cysteine point mutants (4) were also expressed in E. coli and were then reacted with 3 to generate the corresponding disulfide-directed ubiquitinated derivates. B) Primary sequence of α-syn with all sites of ubiquitination noted in red.
Figure 2
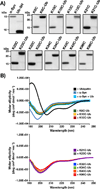
Characterization of ubiquitinated α-synuclein. A) Purified α-synuclein (α-Syn), ubiquitin C-terminal thiol (Ub-SH, 2), α-synuclein lysine to cysteine point mutants (K#C), and the corresponding ubiquitinated derivatives (K#C-Ub) were separated by SDS-PAGE and analyzed by Coomassie staining. B) CD spectra of the same proteins.
Figure 3
Aggregation of ubiquitinated α-synuclein. Purified α-synuclein (α-Syn) and the disulfide-directed ubiquitinated derivatives (K#C-Ub) at a concentration of 100 µM were incubated at 37 °C before analysis by ThT fluorescence (450 nm Ex/482 nm Em) at the indicated timepoints. The same protein samples at day 5 were analyzed by TEM; scale bar: 500 nm). The experiments were performed in triplicate and error bars represent standard deviation.
Similar articles
- Site-specific differences in proteasome-dependent degradation of monoubiquitinated α-synuclein.
Abeywardana T, Lin YH, Rott R, Engelender S, Pratt MR. Abeywardana T, et al. Chem Biol. 2013 Oct 24;20(10):1207-13. doi: 10.1016/j.chembiol.2013.09.009. Chem Biol. 2013. PMID: 24210006 Free PMC article. - Towards elucidation of the role of ubiquitination in the pathogenesis of Parkinson's disease with semisynthetic ubiquitinated α-synuclein.
Hejjaoui M, Haj-Yahya M, Kumar KS, Brik A, Lashuel HA. Hejjaoui M, et al. Angew Chem Int Ed Engl. 2011 Jan 10;50(2):405-9. doi: 10.1002/anie.201005546. Angew Chem Int Ed Engl. 2011. PMID: 21154793 No abstract available. - Ubiquitin ligase Nedd4 promotes alpha-synuclein degradation by the endosomal-lysosomal pathway.
Tofaris GK, Kim HT, Hourez R, Jung JW, Kim KP, Goldberg AL. Tofaris GK, et al. Proc Natl Acad Sci U S A. 2011 Oct 11;108(41):17004-9. doi: 10.1073/pnas.1109356108. Epub 2011 Sep 27. Proc Natl Acad Sci U S A. 2011. PMID: 21953697 Free PMC article. - Molecular mechanisms of alpha-synuclein neurodegeneration.
Waxman EA, Giasson BI. Waxman EA, et al. Biochim Biophys Acta. 2009 Jul;1792(7):616-24. doi: 10.1016/j.bbadis.2008.09.013. Epub 2008 Oct 9. Biochim Biophys Acta. 2009. PMID: 18955133 Free PMC article. Review. - Alteration of Structure and Aggregation of α-Synuclein by Familial Parkinson's Disease Associated Mutations.
Sahay S, Ghosh D, Singh PK, Maji SK. Sahay S, et al. Curr Protein Pept Sci. 2017;18(7):656-676. doi: 10.2174/1389203717666160314151706. Curr Protein Pept Sci. 2017. PMID: 26972727 Review.
Cited by
- A clickable glutamine (CliQ) derivative for the traceless reversible modification of peptides and proteins.
Whedon SD , Parker MK , Tyson EL , Ritterhoff T , Shelton PMM , Chatterjee C . Whedon SD , et al. Chem Commun (Camb). 2019 Feb 12;55(14):2043-2045. doi: 10.1039/c8cc09404g. Chem Commun (Camb). 2019. PMID: 30687853 Free PMC article. - Intrinsic site-selectivity of ubiquitin dimer formation.
Andersen KA, Martin LJ, Prince JM, Raines RT. Andersen KA, et al. Protein Sci. 2015 Feb;24(2):182-9. doi: 10.1002/pro.2603. Epub 2015 Jan 15. Protein Sci. 2015. PMID: 25401704 Free PMC article. - Chemical ubiquitination for decrypting a cellular code.
Stanley M, Virdee S. Stanley M, et al. Biochem J. 2016 May 15;473(10):1297-314. doi: 10.1042/BJ20151195. Biochem J. 2016. PMID: 27208213 Free PMC article. Review. - Chaperones and Proteostasis: Role in Parkinson's Disease.
Joshi N, Raveendran A, Nagotu S. Joshi N, et al. Diseases. 2020 Jun 22;8(2):24. doi: 10.3390/diseases8020024. Diseases. 2020. PMID: 32580484 Free PMC article. Review. - Role of α-synuclein in inducing innate and adaptive immunity in Parkinson disease.
Allen Reish HE, Standaert DG. Allen Reish HE, et al. J Parkinsons Dis. 2015;5(1):1-19. doi: 10.3233/JPD-140491. J Parkinsons Dis. 2015. PMID: 25588354 Free PMC article. Review.
References
- Goedert M. Nat Rev Neurosci. 2001;2:492–501. - PubMed
- Oueslati A, Fournier M, Lashuel HA. Prog Brain Res. 2010;183C:115–145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources