Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex - PubMed (original) (raw)
Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex
Eunice Y Yuen et al. Neuron. 2012.
Abstract
Chronic stress could trigger maladaptive changes associated with stress-related mental disorders; however, the underlying mechanisms remain elusive. In this study, we found that exposing juvenile male rats to repeated stress significantly impaired the temporal order recognition memory, a cognitive process controlled by the prefrontal cortex (PFC). Concomitantly, significantly reduced AMPAR- and NMDAR-mediated synaptic transmission and glutamate receptor expression were found in PFC pyramidal neurons from repeatedly stressed animals. All these effects relied on activation of glucocorticoid receptors and the subsequent enhancement of ubiquitin/proteasome-mediated degradation of GluR1 and NR1 subunits, which was controlled by the E3 ubiquitin ligase Nedd4-1 and Fbx2, respectively. Inhibition of proteasomes or knockdown of Nedd4-1 and Fbx2 in PFC prevented the loss of glutamatergic responses and recognition memory in stressed animals. Our results suggest that repeated stress dampens PFC glutamatergic transmission by facilitating glutamate receptor turnover, which causes the detrimental effect on PFC-dependent cognitive processes.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
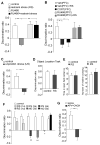
Figure 1. Rats exposed to repeated stress or infused with glutamate receptor antagonists to PFC exhibit worse performance on the temporal order recognition (TOR) memory task
(A) Bar graphs showing the discrimination ratio (DR) of TOR tasks in control groups vs. animals exposed to 7-day restraint stress without or with RU486 injection (10mg/kg, i.p. daily at 30 min before stress). **: p<0.001, ANOVA. (B) Bar graphs showing the DR of TOR tasks in control groups vs. stressed animals (restraint, 7d) with PFC infusion of vehicle or RU486 (1.4 nmol/g, daily at 40 min before stress). Another group of animals was given repeated injections of CORT to the PFC (0.87 nmol/g, 7d). *: p<0.01; #: p<0.05, ANOVA. (C) Bar graphs showing the DR of TOR tasks in control groups vs. animals exposed to 7-day unpredictable stress. **: p<0.001, t test. (D) Bar graphs showing the DR of object location tasks in control groups vs. animals exposed to 7-day restraint stress. (E) Bar graphs showing the time spent at the center in open-field tests and the number of midline crossing in control vs. stressed (restraint, 5d) rats. (F) Bar graphs showing the DR of TOR tasks in control groups, stressed animals (restraint for 1, 3, 5, 7d), and animals withdrawn (WD, for 3 or 5d) from 7-day restraint stress. **: p<0.001; *: p<0.01, t test. (G) Bar graphs showing the DR of TOR tasks in animals with PFC infusion of saline vs. glutamate receptor antagonists (APV: 1 mM, CNQX: 0.2 mM, 1 μl each side). The infusion was performed via an implanted cannula at 20 min before behavioral experiments. **: p<0.001, t test.
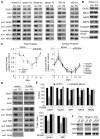
Figure 2. Repeated stress impairs glutamatergic transmission in PFC pyramidal neurons via a post-synaptic mechanism
(A, B) Summarized input-output curves of AMPAR-EPSC (A) or NMDAR-EPSC (B) in response to a series of stimulation intensity in control vs. animals exposed to 7-day repeated restraint stress (RS) or unpredictable stress (US). *: p<0.01, #: p<0.05, ANOVA. Inset: representative EPSC traces. Scale bars: 50pA, 20ms (A) or 100ms (B). (C) Plot of PPR of AMPAR-EPSC and NMDAR-EPSC evoked by double pulses with various intervals in control or stressed rats. (D, E) Cumulative distribution and bar graphs showing the effect of repeated stress on mEPSC amplitude and frequency. *: p<0.01, ANOVA. Inset (D): representative mEPSC traces. Scale bars: 10pA, 1s. (F) Dot plots summarizing the AMPAR, NMDAR and VDCC current density in PFC neurons acutely dissociated from control vs. stressed animals. Inset: representative current traces. Scale bars: 100pA, 1s (AMPA, NMDA) or 2ms (VDCC). (G) Dot plots showing the amplitude of AMPAR-EPSC and NMDAR-EPSC in PFC pyramidal neurons taken from control or stressed animals (restraint, 7-day) with systemic injections of RU486 (10mg/kg). Inset: representative EPSC traces. Scale bars: 50pA, 20ms (AMPA) or 100ms (NMDA). (H) Dot plots showing the amplitude of AMPAR-EPSC in control or stressed animals (restraint, 7-day) with local injections of RU486 (1.4 nmol/g, 7d) to the PFC. (I) Dot plots showing the amplitude of AMPAR-EPSC in animals with local injections of CORT (0.87 nmol/g, 7d) or vehicle control to the PFC. Inset (H, I): representative AMPAR-EPSC traces. Scale bars: 50pA, 20ms. (J) Bar graphs demonstrating the bi-phasic effect of stress on AMPAR-EPSC in rats exposed to various durations of restraint stress.*: p<0.01, ANOVA. Inset: representative AMPAR-EPSC traces. Scale bars: 25pA, 20ms. (K) Dot plots showing the AMPAR-EPSC amplitude in PFC pyramidal neurons, striatal medium spiny neurons and hippocampal CA1 pyramidal neurons from control or stressed rats (restraint, 7-day).
Figure 3. Repeated stress decreases the total and surface levels of AMPAR and NMDAR subunits in PFC through GR activation
(A, C) Immunoblots (A) and quantification analysis (C) of the total and surface AMPAR and NMDAR subunits in PFC from control (con) vs. rats exposed to 1–7 day of restraint stress (RS). Some animals were withdrawn (WD) for different durations (3 or 5 day) after being exposed to 7-day restraint stress. #: p<0.05; *: p<0.01, t test. (B) Immunoblots of the total proteins in PFC from control vs. repeatedly stressed (7-day restraint) rats. (D, E) Immunoblots (D) and quantification analysis (E) of the total and surface AMPAR and NMDAR subunits in PFC from control vs. repeatedly stressed animals without or with RU486 injection (10mg/kg). *: p<0.01, t test. (F) Immunoblots of total GluR1 and NR1 in PFC, striatum and hippocampus from control vs. repeatedly stressed (7-day restraint) rats.
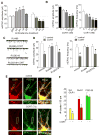
Figure 4. In vitro chronic CORT treatment reduces AMPAR synaptic currents and synaptic GluR1 clusters via GR activation
(A, B) Bar graphs showing the effect of different durations (A) and concentrations (B) of CORT on mEPSC. *: p<0.01, #: p<0.05, ANOVA. (C, D) Representative mEPSC traces (C) and statistic summary (D) showing the effect of CORT (100 nM, 7-day) on mEPSC amplitude and frequency in the presence of GR or MR antagonists in cultured PFC neurons (DIV28–30). Scale bars: 50pA, 1s. *: p<0.01, #: p<0.05, t test. (E) Immunostaining of total GluR1 and PSD-95 in PFC cultures treated with or without CORT (100 nM, 7-day). (F) Bar graphs showing the cluster density of synaptic GluR1 (co-localized, yellow puncta), total GluR1 (red puncta) and PSD-95 (green puncta) in response to CORT treatment. *: p<0.01, t test.
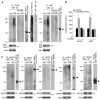
Figure 5. Repeated stress increases the ubiquitination level of GluR1 and NR1 subunits
(A, B) Representative blots (A) and quantification (B) showing the ubiquitination of GluR1 and NR1 subunits in control vs. stressed (7-day restraint) animals without or with RU486 injection (10 mg/kg). *: p<0.01, t test. Lysates of PFC slices were immunoprecipitated with an antibody against GluR1 or NR1, and then blotted with a ubiquitin antibody. Also shown are the input control, the immunoprecipitation control, and the immunoblots of total proteins in control vs. stressed animals. Note, in stressed rats, the immunoprecipitated GluR1 or NR1 showed ubiquitin staining at a molecular mass heavier than the unmodified protein itself. The ladder of ubiquitinated GluR1 or NR1 is typical of proteins that are polyubiquitinated to signal their degradation. (C) Ubiquitination of GluR2, NR2A, NR2B, SAP97 and PSD-95 in control vs. stressed (7-day restraint) animals.
Figure 6. Infusion of a proteasome inhibitor into PFC prevents the loss of glutamate receptors and recognition memory by repeated stress
(A, B) Summarized input-output curves of AMPAR-EPSC (A) or NMDAR-EPSC (B) in control vs. repeatedly stressed (7-day restraint) animals with local injection of the proteasome inhibitor MG132 or saline control. *: p<0.01, #: p<0.05, ANOVA. Inset: representative EPSC traces. Scale bars: 50pA, 20ms (A); 50pA, 100ms (B). (C, D) Representative mEPSC traces and bar graph summary of mEPSC amplitude and frequency in control vs. repeatedly stressed animals with PFC infusion of MG132 or saline. *: p<0.01, t test. Scale bars (C): 25pA, 1s. (E) Bar graphs showing the effect of CORT (100 nM, 7-day) on mEPSC amplitude and frequency in cultured PFC neurons pre-treated with the specific inhibitors of proteasome, lysosome or calpain. *: p<0.01, #: p<0.05, t test. (F, G) Immunoblots and quantification analysis of GluR1 and NR1 expression in control vs. repeatedly stressed animals with PFC infusion of MG132 or saline. *: p<0.01, t test. (H) Quantification analysis of GluR1 expression in control vs. CORT (100 nM, 7-day)-treated PFC cultures pre-incubated without or with proteasome inhibitors. *: p<0.01, t test. (I, J) Bar graphs showing the discrimination ratio (I) and total exploration time (J) of TOR tasks in control groups vs. repeatedly stressed animals (7-day restraint) with stereotaxic injections of saline or MG132 into PFC via an implanted cannula. **: p<0.001, ANOVA.
Figure 7. The E3 ubiquitin ligases Nedd4-1 and Fbx2 are involved in the downregulation of AMPAR- and NMDAR-mediated synaptic reponses by long-term CORT treatment or repeated stress
(A) Representative Western blots in HEK293 cells transfected with HA-tagged rat Nedd4-1 or Fbx2 in the absence or presence of Nedd4-1 shRNA or Fbx2 shRNA. (B, C) Summary data (mean ± SEM) showing the mEPSC amplitude and frequency in control vs. CORT (100 nM, 7d)-treated PFC neurons transfected with Nedd4-1 shRNA, Fbx2 shRNA or GFP control. *: p<0.01, #: p<0.05, t test. (D) Representative mEPSC traces in control vs. CORT-treated PFC neurons with different transfections. Scale bar: 20 pA, 1 sec. (E) Summary data (mean ± SEM) showing the NMDAR current density in control vs. CORT (100 nM, 7d)-treated PFC neurons transfected with Fbx2 shRNA, Nedd4-1 shRNA or GFP control. *: p<0.01, t test. (F) Representative NMDAR currents in control vs. CORT-treated PFC neurons with different transfections. Scale bar: 200 pA, 1 sec. (G, H) Summarized input-output curves of AMPAR-EPSC (G) or NMDAR-EPSC (H) in control vs. repeatedly stressed (7-day restraint) rats with the PFC injection of Nedd4-1 shRNA lentivirus (G), Fbx2 shRNA lentivirus (H), or GFP lentivirus control. *: p<0.01, ANOVA.
Figure 8. Nedd4-1 and Fbx2 are involved in the stress-induced ubiquitination/degradation of GluR1 and NR1 subunits and impairment of recognition memory, and they show differential expression in various brain regions of rats with or without stress exposure
(A, B) Representative blots (A) and quantification (B) showing the ubiquitination and expression of GluR1 and NR1 subunits in control vs. stressed (7-day restraint) animals with PFC injection of GFP lentivirus, Nedd4-1 shRNA lentivirus or Fbx2 shRNA lentivirus *: p<0.01, t test. (C, D) Bar graphs showing the discrimination ratio (C) and total exploration time (D) of TOR tasks in control groups vs. repeatedly stressed animals (7-day restraint) with PFC injection of GFP lentivirus or Nedd4-1 shRNA+Fbx2 shRNA lentiviruses. **: p<0.001, *: p<0.01, ANOVA. (E, F) Representative Western blots and quantification showing the expression of Nedd4-1 and Fbx2 in PFC, striatum and hippocampus of control vs. repeatedly stressed (RS) rats. Actin was used as the loading control. *: p<0.01, ANOVA.
Similar articles
- Histone Modification of Nedd4 Ubiquitin Ligase Controls the Loss of AMPA Receptors and Cognitive Impairment Induced by Repeated Stress.
Wei J, Xiong Z, Lee JB, Cheng J, Duffney LJ, Matas E, Yan Z. Wei J, et al. J Neurosci. 2016 Feb 17;36(7):2119-30. doi: 10.1523/JNEUROSCI.3056-15.2016. J Neurosci. 2016. PMID: 26888924 Free PMC article. - Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition.
Wei J, Yuen EY, Liu W, Li X, Zhong P, Karatsoreos IN, McEwen BS, Yan Z. Wei J, et al. Mol Psychiatry. 2014 May;19(5):588-98. doi: 10.1038/mp.2013.83. Epub 2013 Jul 9. Mol Psychiatry. 2014. PMID: 23835908 - Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory.
Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z. Yuen EY, et al. Mol Psychiatry. 2011 Feb;16(2):156-70. doi: 10.1038/mp.2010.50. Epub 2010 May 11. Mol Psychiatry. 2011. PMID: 20458323 Free PMC article. - Stress-induced deficits in cognition and emotionality: a role of glutamate.
Graybeal C, Kiselycznyk C, Holmes A. Graybeal C, et al. Curr Top Behav Neurosci. 2012;12:189-207. doi: 10.1007/7854_2011_193. Curr Top Behav Neurosci. 2012. PMID: 22261703 Free PMC article. Review. - Stress-induced impairments in prefrontal-mediated behaviors and the role of the N-methyl-D-aspartate receptor.
Graybeal C, Kiselycznyk C, Holmes A. Graybeal C, et al. Neuroscience. 2012 Jun 1;211:28-38. doi: 10.1016/j.neuroscience.2012.02.042. Epub 2012 Feb 28. Neuroscience. 2012. PMID: 22414923 Free PMC article. Review.
Cited by
- An emerging role for microglia in stress-effects on memory.
Sanguino-Gómez J, Buurstede JC, Abiega O, Fitzsimons CP, Lucassen PJ, Eggen BJL, Lesuis SL, Meijer OC, Krugers HJ. Sanguino-Gómez J, et al. Eur J Neurosci. 2022 May;55(9-10):2491-2518. doi: 10.1111/ejn.15188. Epub 2021 May 4. Eur J Neurosci. 2022. PMID: 33724565 Free PMC article. Review. - Positive modulation of NMDA receptors by AGN-241751 exerts rapid antidepressant-like effects via excitatory neurons.
Pothula S, Liu RJ, Wu M, Sliby AN, Picciotto MR, Banerjee P, Duman RS. Pothula S, et al. Neuropsychopharmacology. 2021 Mar;46(4):799-808. doi: 10.1038/s41386-020-00882-7. Epub 2020 Oct 15. Neuropsychopharmacology. 2021. PMID: 33059355 Free PMC article. - Low corticosterone levels attenuate late life depression and enhance glutamatergic neurotransmission in female rats.
Chu SF, Zhang Z, Zhou X, He WB, Yang B, Cui LY, He HY, Wang ZZ, Chen NH. Chu SF, et al. Acta Pharmacol Sin. 2021 Jun;42(6):848-860. doi: 10.1038/s41401-020-00536-w. Epub 2020 Oct 7. Acta Pharmacol Sin. 2021. PMID: 33028984 Free PMC article. - Variations in the nature of behavioral experience can differentially alter the consequences of developmental exposures to lead, prenatal stress, and the combination.
Cory-Slechta DA, Merchant-Borna K, Allen JL, Liu S, Weston D, Conrad K. Cory-Slechta DA, et al. Toxicol Sci. 2013 Jan;131(1):194-205. doi: 10.1093/toxsci/kfs260. Epub 2012 Aug 28. Toxicol Sci. 2013. PMID: 22930682 Free PMC article. - Repeated stress induces a pro-inflammatory state, increases amygdala neuronal and microglial activation, and causes anxiety in adult male rats.
Munshi S, Loh MK, Ferrara N, DeJoseph MR, Ritger A, Padival M, Record MJ, Urban JH, Rosenkranz JA. Munshi S, et al. Brain Behav Immun. 2020 Feb;84:180-199. doi: 10.1016/j.bbi.2019.11.023. Epub 2019 Nov 27. Brain Behav Immun. 2020. PMID: 31785394 Free PMC article.
References
- Alfarez DN, Joëls M, Krugers HJ. Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur J Neurosci. 2003;17:1928–1934. - PubMed
- Andreasen NC, O’Leary DS, Flaum M, Nopoulos P, Watkins GL, Boles Ponto LL, Hichwa RD. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. Lancet. 1997;349:1730–1734. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH085774/MH/NIMH NIH HHS/United States
- MH84233/MH/NIMH NIH HHS/United States
- R25 MH077823/MH/NIMH NIH HHS/United States
- MH85774/MH/NIMH NIH HHS/United States
- R01 MH084233/MH/NIMH NIH HHS/United States
- R01 NS048911/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous