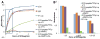Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting - PubMed (original) (raw)
. 2012 Apr 24;6(4):3491-8.
doi: 10.1021/nn300524f. Epub 2012 Mar 14.
Affiliations
- PMID: 22409425
- PMCID: PMC3337349
- DOI: 10.1021/nn300524f
Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting
Lin Zhu et al. ACS Nano. 2012.
Abstract
A novel "smart" multifunctional drug delivery system was successfully developed to respond to the up-regulated matrix metalloprotease 2 (MMP2) in the tumor microenvironment and improve cancer cell-specific delivery of loaded drugs. The system represents a surface-functionalized liposomal nanocarrier, for which two functional polyethylene glycol (PEG)-lipid conjugates were synthesized and characterized. The functionalized liposome was further modified with the tumor cell-specific antinucleosome monoclonal antibody (mAb 2C5). In the resulting system, several drug delivery strategies were combined in the same nanocarrier in a simple way and coordinated in an optimal fashion. The functions of the nanocarrier include (i) the hydrophilic and flexible long PEG chains to prevent nanocarrier nonspecific interactions and prolong its circulation time; (ii) a nanoscale size of the system that allows for its passive tumor targeting via the enhanced permeability and retention (EPR) effect; (iii) a mAb 2C5 to allow for the specific targeting of tumor cells; (iv) a matrix metalloprotease 2-sensitive bond between PEG and lipid that undergoes cleavage in the tumor by the highly expressed extracellular MMP2 for the removal of PEG chains; (v) cell-penetrating peptide (TATp) triggering of the enhanced intracellular delivery of the system after long-chain PEG removal and exposure of the previously hidden surface-attached TATp. It is shown that such a design can enhance the targetability and internalization of nanocarriers in cancer cells.
Figures
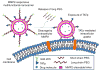
Figure 1. The MMP2-responsive multifunctional liposomal nanocarrier and its drug delivery strategy
The multifunctional liposomal nanocarriers are retained in the tumor site due to the EPR effect and the active targeting effect by the anti-cancer mAb 2C5. The up-regulated MMP2 in the tumor microenvironment cleaves the MMP2-sensitive linker and removes the protective long-chain PEG, resulting in the exposure of TATp for the enhanced cellular internalization.
Figure 2. Characterization of TATp-PEG(2000)-DSPE, MAL-PEG(3400)-peptide and MAL-PEG(3400)-peptide-DOPE
(A), the TLC chromatograms of TATp-PEG(2000)-DSPE. (B) and (C), the RP-HPLC and TLC chromatograms of MAL-PEG(3400)-peptide, respectively. (D), the TLC chromatogram of MAL-PEG(3400)-peptide-DOPE. The developing solvent for TLC was chloroform/methanol (4/1,v/v). The PEG chains were visualized by the Dragendorff’s Reagent. The peptides were visualized by the Ninhydrin Spray Reagent. The phospholipids were visualized by the Molybdenum Blue Spray Reagent. The HPLC chromatogram were collected at 214 nm using gradient solvent conditions: 0–15 min: 5%–75% ACN, 15–15.1 min: 75%–100% ACN, 15.1–20 min: 100% ACN, 20–20.1 min: 100%-5% ACN, 20.1–25 min: 5% ACN, with a flow rate of 1.0 mL/min at room temperature.
Figure 3. Cleavage assays of the MMP2-cleavable peptide and its conjugates
The peptide and its conjugates were treated with the active human MMP2 at two concentrations, 1 and 10 ng/μL, in HBS at 37°C for 24h. The reactions were followed using both RP-HPLC and TLC. Panels A, B, D and E show the RP-HPLC chromatograms of the MMP2-cleavable peptide (A), MAL-PEG(3400)-peptide (B), MAL-PEG(3400)-peptide-DOPE (D), and the mixture of MAL-PEG(3400)-peptide and MAL-PEG(3400)-peptide-DOPE (E). Panels C and F show the TLC chromatograms of MAL-PEG(3400)-peptide (C) and MAL-PEG(3400)-peptide-DOPE (F). The conditions for HPLC and TLC were the same as those in the conjugate characterization section.
Figure 4. Immunological activity of the MMP2-responsive multifunctional liposomal nanocarrier
To study the binding affinities of the liposomal nanocarriers towards nucleosomes, a series of diluted samples were measured using an ELISA assay. The samples included mAb 2C5, IgG, unmodified liposomes (0% Lip), the MMP2-responsive multifunctional nanocarriers incubated at 4°C (2C5/peptide/TATp-Lip 4°C) and at 37°C (2C5/peptide/TATp-Lip 37°C), MMP2-treated MMP2-responsive multifunctional nanocarriers (2C5/peptide/TATp-Lip+MMP2), and MMP2-treated MMP2-responsive multifunctional nanocarriers followed by dialysis (2C5/peptide/TATp-Lip+MMP2+Dia). (A), the UV absorbances of all samples in the ELISA assay. (B), the UV absorbance of the liposomal formulations only in the near linear range from (A). Abbreviation: 2C5, mAb 2C5; peptide, MMP2 cleavable peptide; TATp: TAT peptides; Lip, liposomes; Dia, dialysis.
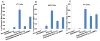
Figure 5. FACS analysis of the interaction of Rh-PE-labeled MMP2-responsive multifunctional liposomal nanocarrier with 4T1 and H9C2 cells
(A), 4T1 cells incubated (for 1h before FACS analysis) with 1% mPEG(2000)-modified liposomes [PEG(2000)-Lip], 1% TATp-modified liposomes (TATp-Lip), and MMP2-responsive nanocarriers [PEG(3400)/peptide/TATp-Lip] with or without pre-treatment with MMP2; (B), H9C2 cells incubated with PEG(2000)-Lip, TATp-Lip, and MMP2-responsive multifunctional nanocarriers (2C5/peptide/TATp-Lip) with or without pre-treatment with MMP2; (C), 4T1 cells incubated with the same liposome preparations as in (B). The geometric mean of the fluorescence for non-treated cells was defined as 100% (*). Abbreviation: 2C5, mAb 2C5; peptide, MMP2 cleavable peptide; TATp: TAT peptides; Lip, liposomes, Rh-PE, 1,2-dioleoylsn- glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (ammonium salt).
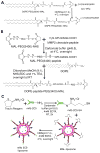
Scheme 1
Synthesis of TATp-PEG(2000)-DSPE (A), MAL-PEG(3400)-peptide-DOPE (B), and surface modification of liposomes with mAb 2C5 (C).
Comment in
- Enhancing the cell uptake of nanoparticles within tumors.
Hui JZ, Al Zaki A, Tsourkas A. Hui JZ, et al. Nanomedicine (Lond). 2012 Jul;7(7):951. Nanomedicine (Lond). 2012. PMID: 23019672 No abstract available.
Similar articles
- Multifunctional PEGylated 2C5-immunoliposomes containing pH-sensitive bonds and TAT peptide for enhanced tumor cell internalization and cytotoxicity.
Koren E, Apte A, Jani A, Torchilin VP. Koren E, et al. J Control Release. 2012 Jun 10;160(2):264-73. doi: 10.1016/j.jconrel.2011.12.002. Epub 2011 Dec 13. J Control Release. 2012. PMID: 22182771 Free PMC article. - Matrix metalloproteinase 2-sensitive multifunctional polymeric micelles for tumor-specific co-delivery of siRNA and hydrophobic drugs.
Zhu L, Perche F, Wang T, Torchilin VP. Zhu L, et al. Biomaterials. 2014 Apr;35(13):4213-22. doi: 10.1016/j.biomaterials.2014.01.060. Epub 2014 Feb 13. Biomaterials. 2014. PMID: 24529391 Free PMC article. - "SMART" drug delivery systems: double-targeted pH-responsive pharmaceutical nanocarriers.
Sawant RM, Hurley JP, Salmaso S, Kale A, Tolcheva E, Levchenko TS, Torchilin VP. Sawant RM, et al. Bioconjug Chem. 2006 Jul-Aug;17(4):943-9. doi: 10.1021/bc060080h. Bioconjug Chem. 2006. PMID: 16848401 Free PMC article. - Environment-responsive multifunctional liposomes.
Kale AA, Torchilin VP. Kale AA, et al. Methods Mol Biol. 2010;605:213-42. doi: 10.1007/978-1-60327-360-2_15. Methods Mol Biol. 2010. PMID: 20072884 Free PMC article. - [Development of a Novel Liposomal DDS by Manipulating Pharmacokinetics and Intracellular Trafficking for Drug Therapy and Nucleic Acid Medicine].
Hatakeyama H. Hatakeyama H. Yakugaku Zasshi. 2018;138(5):591-598. doi: 10.1248/yakushi.17-00206. Yakugaku Zasshi. 2018. PMID: 29709998 Review. Japanese.
Cited by
- Optical imaging of tumor microenvironment.
Wu Y, Zhang W, Li J, Zhang Y. Wu Y, et al. Am J Nucl Med Mol Imaging. 2013;3(1):1-15. Epub 2013 Jan 5. Am J Nucl Med Mol Imaging. 2013. PMID: 23342297 Free PMC article. - Targeted Delivery Methods for Anticancer Drugs.
Veselov VV, Nosyrev AE, Jicsinszky L, Alyautdin RN, Cravotto G. Veselov VV, et al. Cancers (Basel). 2022 Jan 26;14(3):622. doi: 10.3390/cancers14030622. Cancers (Basel). 2022. PMID: 35158888 Free PMC article. Review. - Stimuli-responsive nanocarriers for drug delivery.
Mura S, Nicolas J, Couvreur P. Mura S, et al. Nat Mater. 2013 Nov;12(11):991-1003. doi: 10.1038/nmat3776. Nat Mater. 2013. PMID: 24150417 Review. - Dual Functionalized Liposomes for Selective Delivery of Poorly Soluble Drugs to Inflamed Brain Regions.
Giofrè S, Renda A, Sesana S, Formicola B, Vergani B, Leone BE, Denti V, Paglia G, Groppuso S, Romeo V, Muzio L, Balboni A, Menegon A, Antoniou A, Amenta A, Passarella D, Seneci P, Pellegrino S, Re F. Giofrè S, et al. Pharmaceutics. 2022 Nov 7;14(11):2402. doi: 10.3390/pharmaceutics14112402. Pharmaceutics. 2022. PMID: 36365220 Free PMC article. - Protein-induced supramolecular disassembly of amphiphilic polypeptide nanoassemblies.
Molla MR, Prasad P, Thayumanavan S. Molla MR, et al. J Am Chem Soc. 2015 Jun 17;137(23):7286-9. doi: 10.1021/jacs.5b04285. Epub 2015 Jun 2. J Am Chem Soc. 2015. PMID: 26020143 Free PMC article.
References
- Torchilin VP. Recent Advances with Liposomes as Pharmaceutical Carriers. Nat Rev Drug Discov. 2005;4:145–160. - PubMed
- Iakoubov LZ, Torchilin VP. A Novel Class of Antitumor Antibodies: Nucleosome-Restricted Antinuclear Autoantibodies (ANA) from Healthy Aged Nonautoimmune Mice. Oncol Res. 1997;9:439–446. - PubMed
- Lukyanov AN, Elbayoumi TA, Chakilam AR, Torchilin VP. Tumor-Targeted Liposomes: Doxorubicin-Loaded Long-Circulating Liposomes Modified with Anti-Cancer Antibody. J Control Release. 2004;100:135–144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous