Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation - PubMed (original) (raw)
. 2012 Mar 27;109(13):5004-9.
doi: 10.1073/pnas.1117218109. Epub 2012 Mar 12.
Wen-I Yeh, Patrizia De Sarno, Andrew T Holdbrooks, Yudong Liu, Michelle T Muldowney, Stephanie L Reynolds, Lora L Yanagisawa, Thomas H Fox 3rd, Keun Park, Laurie E Harrington, Chander Raman, Etty N Benveniste
Affiliations
- PMID: 22411837
- PMCID: PMC3323949
- DOI: 10.1073/pnas.1117218109
Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation
Hongwei Qin et al. Proc Natl Acad Sci U S A. 2012.
Abstract
Suppressor of cytokine signaling (SOCS) proteins are feedback inhibitors of the JAK/STAT pathway. SOCS3 has a crucial role in inhibiting STAT3 activation, cytokine signaling, and inflammatory gene expression in macrophages/microglia. To determine the role of SOCS3 in myeloid cells in neuroinflammation, mice with conditional SOCS3 deletion in myeloid cells (LysMCre-SOCS3(fl/fl)) were tested for experimental autoimmune encephalomyelitis (EAE). The myeloid-specific SOCS3-deficient mice are vulnerable to myelin oligodendrocyte glycoprotein (MOG)-induced EAE, with a severe, nonresolving atypical form of disease. In vivo, enhanced infiltration of inflammatory cells and demyelination is prominent in the cerebellum of myeloid-specific SOCS3-deficient mice, as is enhanced STAT3 signaling and expression of inflammatory cytokines/chemokines and an immune response dominated by Th1 and Th17 cells. In vitro, SOCS3-deficient macrophages exhibit heightened STAT3 activation and are polarized toward the classical M1 phenotype. SOCS3-deficient M1 macrophages provide the microenvironment to polarize Th1 and Th17 cells and induce neuronal death. Furthermore, adoptive transfer of M2 macrophages into myeloid SOCS3-deficient mice leads to delayed onset and reduced severity of atypical EAE by decreasing STAT3 activation, Th1/Th17 cells, and proinflammatory mediators in the cerebellum. These findings indicate that myeloid cell SOCS3 provides protection from EAE through deactivation of neuroinflammatory responses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
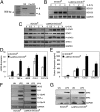
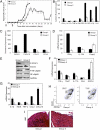
SOCS3 influences M1 polarization of macrophages. (A) SOCS3 floxed mice were cross-bred with B6.129P2-Lyz2tm1(cre)Ifo/J mice to generate deletion of SOCS3 in the myeloid cell lineage. Genomic DNA of BMDMs was distinguished as floxP (fl) and cre-excised alleles (Δ). (B) BMDMs were treated with IL-6 (10 ng/mL) plus sIL-6R (25 ng/mL) for up to 4 h, and mRNA was analyzed by RT-PCR. (C) BMDMs were incubated with IL-6/sIL-6R for up to 4 h, then lysates immunoblotted with the indicated antibodies. (D) BMDMs were incubated with IL-6/sIL-6R for 4 h, and mRNA was analyzed by qRT-PCR. (E) BMDMs were treated with IL-6/sIL-6R for 24 h, cytokine and chemokine protein expression in the supernatants was determined by Multiplex. (F) BMDMs were treated with LPS (10 ng/mL) for 4 h, and mRNA was analyzed by RT-PCR. (G) BMDMs were incubated with LPS for 24 h, then lysates immunoblotted with IRF5, IRF3, and GAPDH Abs. *P < 0.05. Three independent experiments are represented.
Fig. 2.
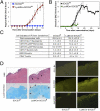
Ablation of SOCS3 in myeloid cells causes atypical EAE. (A) SOCS3fl/fl (n = 13) and LysMCre-SOCS3fl/fl (n = 14) mice were immunized with MOG35-55 peptide. Mean ± SD of atypical EAE scores. (B) Mean ± SD of classical EAE scores. (C) CNS-infiltrating mononuclear cells isolated from the cerebellum of LysMCre-SOCS3fl/fl mice (day 12) and SOCS3fl/fl mice (day 14) after MOG immunization. Cells were stained with trypan blue and counted. Cells stained with Abs to CD4, CD11b, Gr-1, CD11c, Ly-6C, and B220, and the percentage of CD11b+/Gr-1+ neutrophils, CD11b+/Ly-6Chi, and CD11b+/Ly-6Clo monocytes, CD11c+ dendritic cells, B220+ B cells, and CD4+ T cells were gated. The absolute number of cells is shown. **P < 0.001 and *P < 0.05. (D) Sections from the cerebellum of SOCS3fl/fl and LysMCre-SOCS3fl/fl mice (day 13) after MOG35-55 immunization were stained with H&E, LFB, GS-I-B4 for activated macrophages/microglia, CD4 Ab for CD4+ T cells, and myeloperoxidase (MPO) Ab for activated neutrophils. Arrows indicate inflammatory infiltrates and demyelination.
Fig. 3.
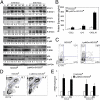
STAT3/4 signaling pathways are aberrantly activated in LysMCre-SOCS3fl/fl mice. (A) Protein extracts from brains of unimmunized (day 0) or MOG35-55–immunized (day 13) SOCS3fl/fl and LysMCre-SOCS3fl/fl mice (n = 5) were immunoblotted with indicated antibodies. (B) Supernatants from brains of MOG-immunized mice (n = 5; day 13) were analyzed for protein expression of CCL2, IL-6, or CXCL10 by Multiplex. *P < 0.05. (C) CNS-infiltrating mononuclear cells from brain at day 13 after MOG immunization. Cells were stimulated with PMA/ionomycin/GolgiStop for 4 h and stained for the surface marker CD4 and intracellular flow for IFN-γ and IL-17A. *P < 0.05, **P < 0.001. (D) Cells were stained for surface markers CD11b and CD45. *P < 0.05. (E) CCL2 and CXCL10 mRNA expression was analyzed by qRT-PCR in CD45int/CD11b+ microglia (Mi) or CD45hi/CD11b+ macrophages/activated microglia (Ma) obtained from the cerebellum. *P < 0.05.
Fig. 4.
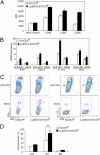
Myeloid cell SOCS3 expression influences Th1 and Th17 differentiation and neuronal viability. (A) BMDMs were incubated with LPS (10 ng/mL) plus IFN-γ (10 ng/mL) for 48 h, then expression of MHC class II, CD40, CD80, or CD86 was examined. *P < 0.05. (B) M1 macrophages and 2D2 CD4+ T cells were cultured at a 1:5 ratio for anti-CD3 or MOG-specific T-cell differentiation. Th1 cells were differentiated with IL-12 (10 ng/mL) and anti–IL-4 (10 μg/mL), and Th17 cells were differentiated with TGF-β (5 ng/mL), IL-6 (20 ng/mL), IL-23 (10 ng/mL), anti–IFN-γ (10 μg/mL), and anti–IL-4 (10 μg/mL). At 4 d, mRNA was extracted from cells and analyzed by qRT-PCR. *P < 0.05. (C) At day 4, cells were analyzed with intracellular flow for IFN-γ or IL-17A. *P < 0.05. (D) BMDMs were polarized to M1 with LPS plus IFN-γ or to M2 with IL-4 (10 ng/mL) for 48 h. Supernatant (400 μL) from each condition was added to primary neuron cultures and incubated for 24 h. Neuronal viability was assessed using the lactate dehydrogenase (LDH) release assay. Average percentage of cytotoxicity ± SD of three independent experiments are shown. *P < 0.05 comparing WT-M1 vs. WT-UN; &P < 0.05 comparing SOCS3−/−M1 vs. SOCS3−/−UN; and #P < 0.05 comparing SOCS3−/−M1 vs. WT-M1.
Fig. 5.
Protective effect of M2 macrophages in atypical EAE. (A) EAE was induced with MOG as described in LysMCre-SOCS3fl/fl mice. BMDMs from SOCS3fl/fl (WT) mice were cultured with 10 ng/mL of M-CSF for 5 d. SOCS3fl/fl M2 macrophages (5 × 106) were transferred by i.v. injection into LysMCre-SOCS3fl/fl mice. Group I (◆), no cells, PBS; group II (☐), cell transfer 1 d before MOG and 3 d after MOG injection (black arrows). Development of atypical EAE was compared between the two groups (n = 3). Protein expression levels of cytokines (B) and chemokines (C) was determined by Multiplex from brains of group I or group II mice (days 13 and 28), respectively. *P < 0.05. (D) mRNA from group 1 or group II brains (days 13 and 28), respectively, was analyzed by qRT-PCR. *P < 0.05. (E) In a separate experiment, brain homogenates from group I (n = 5) and group II (n = 5) (day 18) were immunoblotted with the indicated antibodies (F and G). mRNA from group 1 or group II brain (day 18) was analyzed by qRT-PCR. *P < 0.05. (H) CNS-infiltrating mononuclear cells from brains of group I and group II mice 18 d after MOG immunization and CD11b and CD45 expression determined by flow cytometry. (I) Sections from LysMCre-SOCS3fl/fl mice stained by H&E (day 18).
Similar articles
- Preferential Recruitment of Neutrophils into the Cerebellum and Brainstem Contributes to the Atypical Experimental Autoimmune Encephalomyelitis Phenotype.
Liu Y, Holdbrooks AT, Meares GP, Buckley JA, Benveniste EN, Qin H. Liu Y, et al. J Immunol. 2015 Aug 1;195(3):841-52. doi: 10.4049/jimmunol.1403063. Epub 2015 Jun 17. J Immunol. 2015. PMID: 26085687 Free PMC article. - SOCS3 deficiency promotes M1 macrophage polarization and inflammation.
Qin H, Holdbrooks AT, Liu Y, Reynolds SL, Yanagisawa LL, Benveniste EN. Qin H, et al. J Immunol. 2012 Oct 1;189(7):3439-48. doi: 10.4049/jimmunol.1201168. Epub 2012 Aug 27. J Immunol. 2012. PMID: 22925925 Free PMC article. - Therapeutic efficacy of suppressing the Jak/STAT pathway in multiple models of experimental autoimmune encephalomyelitis.
Liu Y, Holdbrooks AT, De Sarno P, Rowse AL, Yanagisawa LL, McFarland BC, Harrington LE, Raman C, Sabbaj S, Benveniste EN, Qin H. Liu Y, et al. J Immunol. 2014 Jan 1;192(1):59-72. doi: 10.4049/jimmunol.1301513. Epub 2013 Dec 9. J Immunol. 2014. PMID: 24323580 Free PMC article. - The roles of SOCS3 and STAT3 in bacterial infection and inflammatory diseases.
Gao Y, Zhao H, Wang P, Wang J, Zou L. Gao Y, et al. Scand J Immunol. 2018 Dec;88(6):e12727. doi: 10.1111/sji.12727. Scand J Immunol. 2018. PMID: 30341772 Review. - SOCS3 and STAT3, major controllers of the outcome of infection with Mycobacterium tuberculosis.
Rottenberg ME, Carow B. Rottenberg ME, et al. Semin Immunol. 2014 Dec;26(6):518-32. doi: 10.1016/j.smim.2014.10.004. Epub 2014 Nov 1. Semin Immunol. 2014. PMID: 25458989 Review.
Cited by
- Inhibition of the JAK/STAT Pathway Protects Against α-Synuclein-Induced Neuroinflammation and Dopaminergic Neurodegeneration.
Qin H, Buckley JA, Li X, Liu Y, Fox TH 3rd, Meares GP, Yu H, Yan Z, Harms AS, Li Y, Standaert DG, Benveniste EN. Qin H, et al. J Neurosci. 2016 May 4;36(18):5144-59. doi: 10.1523/JNEUROSCI.4658-15.2016. J Neurosci. 2016. PMID: 27147665 Free PMC article. - Sigma 1 Receptor Contributes to Astrocyte-Mediated Retinal Ganglion Cell Protection.
Zhao J, Gonsalvez GB, Mysona BA, Smith SB, Bollinger KE. Zhao J, et al. Invest Ophthalmol Vis Sci. 2022 Feb 1;63(2):1. doi: 10.1167/iovs.63.2.1. Invest Ophthalmol Vis Sci. 2022. PMID: 35103752 Free PMC article. - Goblet cell-produced retinoic acid suppresses CD86 expression and IL-12 production in bone marrow-derived cells.
Xiao Y, de Paiva CS, Yu Z, de Souza RG, Li DQ, Pflugfelder SC. Xiao Y, et al. Int Immunol. 2018 Sep 25;30(10):457-470. doi: 10.1093/intimm/dxy045. Int Immunol. 2018. PMID: 30010888 Free PMC article. - Deficiency of Socs3 leads to brain-targeted EAE via enhanced neutrophil activation and ROS production.
Yan Z, Yang W, Parkitny L, Gibson SA, Lee KS, Collins F, Deshane JS, Cheng W, Weinmann AS, Wei H, Qin H, Benveniste EN. Yan Z, et al. JCI Insight. 2019 Apr 2;5(9):e126520. doi: 10.1172/jci.insight.126520. JCI Insight. 2019. PMID: 30939124 Free PMC article. - Beneficial or Harmful Role of Macrophages in Guillain-Barré Syndrome and Experimental Autoimmune Neuritis.
Shen D, Chu F, Lang Y, Geng Y, Zheng X, Zhu J, Liu K. Shen D, et al. Mediators Inflamm. 2018 Apr 26;2018:4286364. doi: 10.1155/2018/4286364. eCollection 2018. Mediators Inflamm. 2018. PMID: 29853789 Free PMC article. Review.
References
- Lopez-Diego RS, Weiner HL. Novel therapeutic strategies for multiple sclerosis—a multifaceted adversary. Nat Rev Drug Discov. 2008;7:909–925. - PubMed
- Ponomarev ED, Shriver LP, Maresz K, Dittel BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res. 2005;81:374–389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS45290/NS/NINDS NIH HHS/United States
- P30 NS057098/NS/NINDS NIH HHS/United States
- P30 AR48311/AR/NIAMS NIH HHS/United States
- P30 AR048311/AR/NIAMS NIH HHS/United States
- R01 AI076562/AI/NIAID NIH HHS/United States
- R01 NS057563/NS/NINDS NIH HHS/United States
- NS64261/NS/NINDS NIH HHS/United States
- NS47466/NS/NINDS NIH HHS/United States
- R01 DK084082/DK/NIDDK NIH HHS/United States
- R01 NS064261/NS/NINDS NIH HHS/United States
- AI76562/AI/NIAID NIH HHS/United States
- P30 NS047466/NS/NINDS NIH HHS/United States
- P30 AI027767/AI/NIAID NIH HHS/United States
- P30 AI27767/AI/NIAID NIH HHS/United States
- R01 NS045290/NS/NINDS NIH HHS/United States
- DK84082/DK/NIDDK NIH HHS/United States
- CA 1059-A-13/CA/NCI NIH HHS/United States
- NS57098/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous