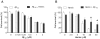Interactions between amyloid-β and hemoglobin: implications for amyloid plaque formation in Alzheimer's disease - PubMed (original) (raw)
Interactions between amyloid-β and hemoglobin: implications for amyloid plaque formation in Alzheimer's disease
Jia-Ying Chuang et al. PLoS One. 2012.
Abstract
Accumulation of amyloid-β (Aβ) peptides in the brain is one of the central pathogenic events in Alzheimer's disease (AD). However, why and how Aβ aggregates within the brain of AD patients remains elusive. Previously, we demonstrated hemoglobin (Hb) binds to Aβ and co-localizes with the plaque and vascular amyloid deposits in post-mortem AD brains. In this study, we further characterize the interactions between Hb and Aβ in vitro and in vivo and report the following observations: 1) the binding of Hb to Aβ required iron-containing heme; 2) other heme-containing proteins, such as myoglobin and cytochrome C, also bound to Aβ; 3) hemin-induced cytotoxicity was reduced in neuroblastoma cells by low levels of Aβ; 4) Hb was detected in neurons and glial cells of post-mortem AD brains and was up-regulated in aging and APP/PS1 transgenic mice; 5) microinjection of human Hb into the dorsal hippocampi of the APP/PS1 transgenic mice induced the formation of an envelope-like structure composed of Aβ surrounding the Hb droplets. Our results reveal an enhanced endogenous expression of Hb in aging brain cells, probably serving as a compensatory mechanism against hypoxia. In addition, Aβ binds to Hb and other hemoproteins via the iron-containing heme moiety, thereby reducing Hb/heme/iron-induced cytotoxicity. As some of the brain Hb could be derived from the peripheral circulation due to a compromised blood-brain barrier frequently observed in aged and AD brains, our work also suggests the genesis of some plaques may be a consequence of sustained amyloid accretion at sites of vascular injury.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
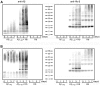
Figure 1. Interaction between hemoglobin (Hb) and Aβ.
Freshly dissolved Aβ peptides (50 µM) were incubated with an equal mass of Hb (3.5 µM) at 37°C for 0, 1, 3 and 7 d. A) Aβ1–40; B) Aβ1–42. Left panels, anti-Aβ; right panels, anti-Hb-β chain.
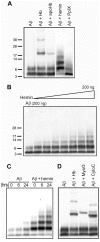
Figure 2. Heme is the Aβ binding moiety of hemoglobin (Hb).
A) Interactions between Aβ and various part of Hb. B) Hemin induced the Aβ oligomerization in a dose-dependent fashion. C) Hemin induced the Aβ oligomerization in a time-dependent fashion. Incubation times: 0, 6, 24 hours. D) Aβ binds to Hb, myoglobin (MyoG) and cytochrome C (CytoC).
Figure 3. Effects of Aβ on hemin induced cytotoxicity.
A) Differentiated neuroblastoma cells, SH-SY5Y, were treated with different doses of pre-incubated Aβ1–42 without (open bars) or with 0.25 µM of hemin (closed bars). B) Differentiated SH-SY5Y cells were treated with different doses of hemin without (open bars) or with 0.25 µM of pre-incubated Aβ1–42 (closed bars). The cell viability was determined by MTT reduction assay. Bonferroni's post-hoc test: * p<0.05 vs. respective hemin group.
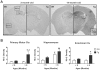
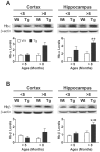
Figure 4. The expression of hemoglobin (Hb) in APP/PS1 transgenic (Tg) mice and wild-type (Wt) littermates.
A) Representative immuno-micrographs reveal the intensities of Hb-β+ stains in the coronal brain sections of a 3-month-old and a 14-month-old Tg and Wt mice, respectively. Insert: enlarged micrograph shows the presence of Hb-β+ stains in a plaque-like structure and some brain cells. B) Quantitative results of the optical densities of Hb-β+ stains in primary motor, hippocampus and entorhinal cortex of <5-month-old and >8-month-old Tg and Wt mice. Bonferroni's post-hoc test: * p<0.05 vs. respective Wt group; # p<0.05, ## p<0.01 vs. respective <5-month-old group.
Figure 5. The levels of hemoglobin (Hb)-α and Hb-β in <5-month-old and >8-month-old APP/PS1 transgenic (Tg) mice and wild-type (Wt) littermates.
Representative immunoblots are shown on the top of each panel whereas quantitative results are shown in the respective lower panel. The levels of Hb monomer (∼16 kDa) are quantified by immunoblotting analyses. Bonferroni's post-hoc test: * p<0.05, ** p<0.01, vs. respective Wt group; ## p<0.01 vs. respective <5-month-old group.
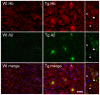
Figure 6. Localization of hemoglobin (Hb) immunoreactivity in brains of APP/PS1 transgenic (Tg) mice.
Double immunofluorescent micrographs reveal the presence of Hb-β+ stains in Aβ+ amyloid plaques and some brain cells. Scale bars: 50 µm. Examples of enlarged micrographs are shown in the right. Arrowheads point to Hb-β+, but APP/Aβ− cells; while, arrows show APP/Aβ+, but Hb-β− cells.
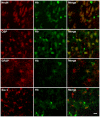
Figure 7. The distribution of hemoglobin (Hb) immunoreactivity in inferior temporal gyrus of post-mortem human brain.
Hb-β+ stains are present in most NeuN+ neurons and OSP+ oligodendrocytes; while only a small fractions of GFAP+ astrocytes and Iba-1+ microglia express Hb. Scale bars: 10 µm.
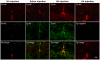
Figure 8. Hemoglobin (Hb) induces Aβ accumulation in the immediate surroundings of Hb.
Human Hb (1 µl, 50 µg/µl) was injected into the dorsal hippocampus of 4-month-old APP/PS1 transgenic (Tg) mice. One week after the injection of Hb or saline, the brain sections were double stained for Hb-β and Aβ. Tg-negative: The sections were stained only by Hb antibodies, but not Aβ antibodies and serve as negative controls for Aβ staining. Scale bars: 30 µm.
Similar articles
- The effect of focal brain injury on beta-amyloid plaque deposition, inflammation and synapses in the APP/PS1 mouse model of Alzheimer's disease.
Collins JM, King AE, Woodhouse A, Kirkcaldie MT, Vickers JC. Collins JM, et al. Exp Neurol. 2015 May;267:219-29. doi: 10.1016/j.expneurol.2015.02.034. Epub 2015 Mar 4. Exp Neurol. 2015. PMID: 25747037 - House dust mite-induced asthma exacerbates Alzheimer's disease changes in the brain of the AppNL-G-F mouse model of disease.
Sahu B, Nookala S, Floden AM, Ambhore NS, Sathish V, Klug MG, Combs CK. Sahu B, et al. Brain Behav Immun. 2024 Oct;121:365-383. doi: 10.1016/j.bbi.2024.07.038. Epub 2024 Jul 29. Brain Behav Immun. 2024. PMID: 39084541 Free PMC article. - Pyroglutamate Abeta pathology in APP/PS1KI mice, sporadic and familial Alzheimer's disease cases.
Wirths O, Bethge T, Marcello A, Harmeier A, Jawhar S, Lucassen PJ, Multhaup G, Brody DL, Esparza T, Ingelsson M, Kalimo H, Lannfelt L, Bayer TA. Wirths O, et al. J Neural Transm (Vienna). 2010 Jan;117(1):85-96. doi: 10.1007/s00702-009-0314-x. Epub 2009 Oct 13. J Neural Transm (Vienna). 2010. PMID: 19823761 Free PMC article. - APP transgenic modeling of Alzheimer's disease: mechanisms of neurodegeneration and aberrant neurogenesis.
Crews L, Rockenstein E, Masliah E. Crews L, et al. Brain Struct Funct. 2010 Mar;214(2-3):111-26. doi: 10.1007/s00429-009-0232-6. Epub 2009 Nov 29. Brain Struct Funct. 2010. PMID: 20091183 Free PMC article. Review. - The role of amyloid in the pathogenesis of Alzheimer's disease.
Verbeek MM, Ruiter DJ, de Waal RM. Verbeek MM, et al. Biol Chem. 1997 Sep;378(9):937-50. doi: 10.1515/bchm.1997.378.9.937. Biol Chem. 1997. PMID: 9348103 Review.
Cited by
- Systemic Inflammation Is Associated With Longitudinal Changes in Cognitive Performance Among Urban Adults.
Beydoun MA, Dore GA, Canas JA, Liang H, Beydoun HA, Evans MK, Zonderman AB. Beydoun MA, et al. Front Aging Neurosci. 2018 Oct 9;10:313. doi: 10.3389/fnagi.2018.00313. eCollection 2018. Front Aging Neurosci. 2018. PMID: 30356710 Free PMC article. - Acquired Resilience: An Evolved System of Tissue Protection in Mammals.
Stone J, Mitrofanis J, Johnstone DM, Falsini B, Bisti S, Adam P, Nuevo AB, George-Weinstein M, Mason R, Eells J. Stone J, et al. Dose Response. 2018 Dec 27;16(4):1559325818803428. doi: 10.1177/1559325818803428. eCollection 2018 Oct-Dec. Dose Response. 2018. PMID: 30627064 Free PMC article. Review. - Candidate SNP Markers of Familial and Sporadic Alzheimer's Diseases Are Predicted by a Significant Change in the Affinity of TATA-Binding Protein for Human Gene Promoters.
Ponomarenko P, Chadaeva I, Rasskazov DA, Sharypova E, Kashina EV, Drachkova I, Zhechev D, Ponomarenko MP, Savinkova LK, Kolchanov N. Ponomarenko P, et al. Front Aging Neurosci. 2017 Jul 20;9:231. doi: 10.3389/fnagi.2017.00231. eCollection 2017. Front Aging Neurosci. 2017. PMID: 28775688 Free PMC article. - Combined assessment of serum folate and hemoglobin as biomarkers of brain amyloid β accumulation.
Yoshinaga T, Nishimata H, Kajiya Y, Yokoyama S. Yoshinaga T, et al. PLoS One. 2017 Apr 13;12(4):e0175854. doi: 10.1371/journal.pone.0175854. eCollection 2017. PLoS One. 2017. PMID: 28406978 Free PMC article. - Twelve protections evolved for the brain, and their roles in extending its functional life.
Stone J, Mitrofanis J, Johnstone DM, Robinson SR. Stone J, et al. Front Neuroanat. 2023 Nov 6;17:1280275. doi: 10.3389/fnana.2023.1280275. eCollection 2023. Front Neuroanat. 2023. PMID: 38020212 Free PMC article. Review.
References
- Evin G, Weidemann A. Biogenesis and metabolism of Alzheimer's disease Abeta amyloid peptides. Peptides. 2002;23:1285–1297. - PubMed
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. - PubMed
- Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. - PubMed
- Hardy J. Alzheimer's disease: the amyloid cascade hypothesis: an update and reappraisal. J Alzheimers Dis. 2006;9:151–153. - PubMed
- Kayed R, Head E, Thompson JL, Mclntire TM, Milton SC, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials