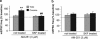Temporally dependent changes in cocaine-induced synaptic plasticity in the nucleus accumbens shell are reversed by D1-like dopamine receptor stimulation - PubMed (original) (raw)
Temporally dependent changes in cocaine-induced synaptic plasticity in the nucleus accumbens shell are reversed by D1-like dopamine receptor stimulation
Pavel I Ortinski et al. Neuropsychopharmacology. 2012 Jun.
Abstract
Dopaminergic and glutamatergic inputs to the nucleus accumbens shell have a central role in reward processing. Non-contingent cocaine administration generates a number of long-term AMPA receptor-dependent changes in synaptic efficacy. However, the synaptic consequences of cocaine self-administration and the potential role of dopamine in these processes remain unclear. Here, we examined the influence of D1 dopamine receptor (D1DR) activation on excitatory synaptic plasticity in the accumbens shell of adult rats following cocaine self-administration. Our results indicated that during the first 2 days following cocaine exposure both pre- and post-synaptic mechanisms contribute to a net decrease in AMPA receptor-mediated signaling. This is reflected by decreased frequency of miniature EPSCs (mEPSCs) attributable to enhanced cannabinoid receptor activity, decreased mEPSC amplitude, and increased paired-pulse ratio of evoked EPSCs. In contrast, the only changes observed in the shell 3-4 weeks following cocaine self-administration were increased mEPSCs amplitudes and AMPA/NMDA ratios. We further found that although these cocaine-induced neuroadaptations during early and late abstinence have different synaptic expression mechanisms, they were normalized by stimulation of D1DRs. Thus, pre-exposure to the D1DR agonist, SKF38393, during the initial period of abstinence increased excitatory synaptic strength, but reduced excitatory signaling after weeks of abstinence. Taken together, these results indicate that the direction of changes in excitatory transmission induced by cocaine self-administration switches over the first few weeks of abstinence. Moreover, D1DRs gate the stability of these cocaine-induced changes at glutamatergic synapses in the accumbens shell by utilizing multiple temporally distinct mechanisms, which has implications for the treatment of cocaine craving and addiction.
Figures
Figure 1
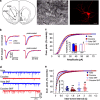
D1DR stimulation increases the mEPSC amplitude and frequency in cocaine-experienced animals at 1–2 days of abstinence. (a) Left, a schematic of a coronal brain section illustrating the two subdivisions of the nucleus accumbens. Shaded box indicates the area of the shell used for all recordings. Middle, an infrared differential interference contrast image of an MSN in the nucleus accumbens shell (open arrow) with the recording electrode (closed arrow) impaling the cell. Right, a confocal microscope image of an accumbens shell MSN filled with Alexa 568. Scale bars, 25 μm. (b) Left, mEPSC traces from cells in cocaine-experienced and yoked saline animals with and without SKF38393 (10 μM) pre-treatment. Right, same traces normalized to the peak amplitude illustrate differences in mEPSC decay. (c) Cumulative probability distributions indicate reduced mEPSC amplitudes in cells from cocaine-experienced rats relative to yoked saline controls, under basal conditions (ie, without SKF38393 pre-treatment; p<0.05, K–S test). SKF38393 pre-treatment results in a rightward shift of the distribution for cells from the cocaine-experienced, but not yoked saline control group. The inset shows mean mEPSC amplitudes for all groups (_n_=12–17 cells/5–6 animals). **t(28)=2.98, p<0.01 vs yoked controls; #t(32)=2.66, p<0.05 vs cocaine no SKF; Student's _t_-test. (d) Representative traces illustrate effects of SKF38393 pre-treatment on mEPSC frequency. (e) Cumulative probability distributions of mEPSC inter-event intervals show reduced frequency of mEPSCs under basal conditions in cocaine-experienced animals relative to yoked saline controls (p<0.01, K–S test). The mEPSCs frequency deficit is absent following SKF38393 pre-treatment. Inset shows the effect of SKF38393 on mEPSC frequency in all recorded cells (black horizontal lines indicate the mean). Mean (±SEM) frequencies are as follows: yoke 7.8±0.9 Hz; cocaine 4.4±0.7 Hz; yoke SKF 8.7±1.6 Hz; cocaine SKF 10±1.4 Hz. Notice that three cells from the yoke SKF group had very frequent events contributing to the leftward shift of the cumulative probability distribution. **t(30)=2.98, p<0.01 vs yoked controls; ##t(32)=3.64, p<0.01 vs cocaine no SKF; Student's _t_-test.
Figure 2
D1DR stimulation decreases the mEPSC amplitude and does not affect frequency in cocaine-experienced animals at 3–4 weeks of abstinence. (a) Left, mEPSC traces from cells in cocaine-experienced and yoked saline animals with and without SKF38393 (10 μM) pre-treatment. Right, same traces normalized to the peak amplitude for comparison of mEPSC decay. (b) Cumulative probability distributions indicate increased basal mEPSC amplitudes in cells from cocaine-experienced rats relative to yoked saline controls (p<0.01, K–S test). SKF38393 pre-treatment only affects the amplitude distribution in the cocaine-experienced group, shifting it toward smaller mEPSC amplitudes. The inset shows mean mEPSC amplitudes for all groups (_n_=13–20 cells/5–7 animals). *t(35)=2.35, p<0.05 vs yoked controls; #t(33)=2.31, p<0.05 vs cocaine no SKF; Student's _t_-test. (c) Representative traces illustrate the effects of SKF38393 pre-treatment on mEPSC frequency. (d) Cumulative probability distributions of mEPSC inter-event intervals and scatterplots of mEPSC frequencies (inset). Mean (±SEM) frequencies are as follows: yoke 5.1±0.7 Hz; cocaine 6.1±0.9 Hz; yoke SKF 7.2±1.3 Hz; cocaine SKF 6.5±0.8 Hz. There were no differences between any of the groups in either the cumulative inter-event interval distributions or the mean mEPSC frequencies.
Figure 3
Effects of SKF38393 pre-treatment on PPR in early and late abstinence from cocaine self-administration. (a) Sample paired eEPSC comparing the effects of cocaine exposure and SKF38393 incubation on PPRs in early abstinence from cocaine self-administration. Inter-stimulus interval is 100 ms. Stimulus artifacts are omitted. (b) Same as in (a) but in late abstinence. (c) Mean PPRs measured at inter-stimulus intervals (ISIs) of 20–500 ms at 1–2 days of abstinence from cocaine self-administration. There is a significant main effect of treatment (repeated-measures ANOVA, F(3,44)=3.353, _p_=0.027). Post hocs on the main effect of treatment revealed a significant effect of yoke vs cocaine and cocaine vs cocaine SKF groups (*Tukey's HSD post hoc). In all, 11–15 cells from 4 to 5 animals were recorded in each group. (d) Mean paired pulse ratios at 3–4 weeks of abstinence. Differences between groups are not significant (repeated-measures ANOVA, F(3,39)=1.647, _p_=0.194). 10–12 cells from 4 to 5 animals were recorded in each group.
Figure 4
Pre-synaptic effects of cocaine self-administration and D1 agonist pre-treatment in early abstinence involve modulation of CB1 receptor signaling. (a) Bar histograms illustrate an enhanced effect of AM251 on mEPSC frequency in neurons from cocaine-experienced animals at 1–2 days of abstinence from cocaine self-administration. This effect is suppressed following incubation with SKF38393. (b) Same as (a), but at 3–4 weeks of abstinence. In all, 8–11 cells from 4 to 5 animals were recorded in each group. **t(18)=4.33, p<0.01 vs not treated yoke; ##t(17)=4.24, p<0.01 vs not treated cocaine; Student's _t_-tests.
Figure 5
SKF38393 pre-exposure reverses the cocaine-associated changes in AMPA/NMDA ratio. (a, c) Representative average current traces show AMPA (thin traces) and compound, AMPA+NMDA (thick traces) receptor-mediated eEPSCs from yoked saline and cocaine-experienced groups with and without SKF38393 pre-treatment in early (a) and late (c) abstinence. Each trace is an average of 50 individual eEPSCs. For display purposes, AMPA receptor-mediated currents are shown as outward-going. Arrows indicate time-points for measurement of the NMDA component (see Subjects and methods). (b, d) Mean AMPA/NMDA ratios in early (b) and late (d) abstinence from cocaine self-administration. In all, 11–15 cells from 4 to 5 animals were recorded in each of the groups. *t(21)=2.61, p<0.05 vs yoke; #t(24)=2.71, p<0.05 vs not treated cocaine; Student's _t_-test.
Figure 6
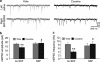
In vivo microinjection of SKF38393 rescues mEPSC amplitude and frequency deficits. (a) Sample mEPSC traces from NAc shell MSNs of yoked saline and cocaine animals following an injection of saline (0.5 μl) into the NAc shell of the left hemisphere and SKF38393 (1 μg/0.5 μl) into the NAc shell of the right hemisphere. Microinjections and recordings were performed 24 h following the last self-administration session (see Subjects and methods for details). (b) Decreased mEPSC amplitudes in saline-treated hemispheres are restored to control levels in the SKF38393-treated hemispheres. **t(19)=2.91, p<0.01; #t(20)=2.65, p<0.05, Student's _t_-tests; C, SKF38393 microinjection rescues the reduction in mEPSC frequency. **t(19)=4.31, p<0.001; #t(20)=2.67, p<0.05; Student's _t_-tests; _n_=10–11 cells from four animals.
Similar articles
- Extrasynaptic targeting of NMDA receptors following D1 dopamine receptor activation and cocaine self-administration.
Ortinski PI, Turner JR, Pierce RC. Ortinski PI, et al. J Neurosci. 2013 May 29;33(22):9451-61. doi: 10.1523/JNEUROSCI.5730-12.2013. J Neurosci. 2013. PMID: 23719812 Free PMC article. - Dual synaptic sites of D(1)-dopaminergic regulation of ethanol sensitivity of NMDA receptors in nucleus accumbens.
Zhang TA, Hendricson AW, Morrisett RA. Zhang TA, et al. Synapse. 2005 Oct;58(1):30-44. doi: 10.1002/syn.20181. Synapse. 2005. PMID: 16037948 - GSK-3β inhibitors reverse cocaine-induced synaptic transmission dysfunction in the nucleus accumbens.
Zhao R, Chen J, Ren Z, Shen H, Zhen X. Zhao R, et al. Synapse. 2016 Nov;70(11):461-70. doi: 10.1002/syn.21922. Epub 2016 Jul 22. Synapse. 2016. PMID: 27377051 - Mechanisms by which dopamine receptors may influence synaptic plasticity.
Wolf ME, Mangiavacchi S, Sun X. Wolf ME, et al. Ann N Y Acad Sci. 2003 Nov;1003:241-9. doi: 10.1196/annals.1300.015. Ann N Y Acad Sci. 2003. PMID: 14684450 Review. - Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration.
Self DW, Choi KH, Simmons D, Walker JR, Smagula CS. Self DW, et al. Learn Mem. 2004 Sep-Oct;11(5):648-57. doi: 10.1101/lm.81404. Learn Mem. 2004. PMID: 15466321 Free PMC article. Review.
Cited by
- Low frequency deep brain stimulation of nucleus accumbens shell neuronal subpopulations attenuates cocaine seeking selectively in male rats.
Swinford-Jackson SE, Rich MT, Huffman PJ, Knouse MC, Thomas AS, Mankame S, Worobey SJ, Pierce RC. Swinford-Jackson SE, et al. Addict Neurosci. 2023 Dec 15;9:100133. doi: 10.1016/j.addicn.2023.100133. Epub 2023 Oct 20. Addict Neurosci. 2023. PMID: 38312329 Free PMC article. - Dopamine Triggers CTCF-Dependent Morphological and Genomic Remodeling of Astrocytes.
Galloway A, Adeluyi A, O'Donovan B, Fisher ML, Rao CN, Critchfield P, Sajish M, Turner JR, Ortinski PI. Galloway A, et al. J Neurosci. 2018 May 23;38(21):4846-4858. doi: 10.1523/JNEUROSCI.3349-17.2018. Epub 2018 Apr 30. J Neurosci. 2018. PMID: 29712779 Free PMC article. - Deletion of Maged1 in mice abolishes locomotor and reinforcing effects of cocaine.
De Backer JF, Monlezun S, Detraux B, Gazan A, Vanopdenbosch L, Cheron J, Cannazza G, Valverde S, Cantacorps L, Nassar M, Venance L, Valverde O, Faure P, Zoli M, De Backer O, Gall D, Schiffmann SN, de Kerchove d'Exaerde A. De Backer JF, et al. EMBO Rep. 2018 Sep;19(9):e45089. doi: 10.15252/embr.201745089. Epub 2018 Jul 12. EMBO Rep. 2018. PMID: 30002119 Free PMC article. - Cocaine induces input and cell-type-specific synaptic plasticity in ventral pallidum-projecting nucleus accumbens medium spiny neurons.
Inbar K, Levi LA, Kupchik YM. Inbar K, et al. Neuropsychopharmacology. 2022 Jul;47(8):1461-1472. doi: 10.1038/s41386-022-01285-6. Epub 2022 Feb 4. Neuropsychopharmacology. 2022. PMID: 35121830 Free PMC article. - CaMKII activity in the ventral tegmental area gates cocaine-induced synaptic plasticity in the nucleus accumbens.
Liu X, Liu Y, Zhong P, Wilkinson B, Qi J, Olsen CM, Bayer KU, Liu QS. Liu X, et al. Neuropsychopharmacology. 2014 Mar;39(4):989-99. doi: 10.1038/npp.2013.299. Epub 2013 Oct 24. Neuropsychopharmacology. 2014. PMID: 24154664 Free PMC article.
References
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, et al. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–353. - PubMed
- Anderson SM, Pierce RC. Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacol Ther. 2005;106:389–403. - PubMed
- Andre VM, Cepeda C, Cummings DM, Jocoy EL, Fisher YE, William Yang X, et al. Dopamine modulation of excitatory currents in the striatum is dictated by the expression of D1 or D2 receptors and modified by endocannabinoids. Eur J Neurosci. 2010;31:14–28. - PubMed
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DA22339/DA/NIDA NIH HHS/United States
- R01 DA022339/DA/NIDA NIH HHS/United States
- K01 DA031747/DA/NIDA NIH HHS/United States
- K02 DA18678/DA/NIDA NIH HHS/United States
- K02 DA018678/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources