Increased dietary sodium is independently associated with greater mortality among prevalent hemodialysis patients - PubMed (original) (raw)
Randomized Controlled Trial
doi: 10.1038/ki.2012.42. Epub 2012 Mar 14.
Affiliations
- PMID: 22418981
- PMCID: PMC3779624
- DOI: 10.1038/ki.2012.42
Randomized Controlled Trial
Increased dietary sodium is independently associated with greater mortality among prevalent hemodialysis patients
Finnian R Mc Causland et al. Kidney Int. 2012 Jul.
Abstract
Dietary sodium is thought to play a major role in the pathogenesis of hypertension, hypervolemia, and mortality in hemodialysis patients; hence, sodium restriction is almost universally recommended. Since the evidence upon which to base these assumptions is limited, we undertook a post-hoc analysis of 1770 patients in the Hemodialysis Study with available dietary, clinical, and laboratory information. Within this cohort, 772 were men, 1113 black, and 786 diabetic, with a mean age of 58 years and a median dietary sodium intake of 2080 mg/day. After case-mix adjustment, linear regression modeling found that higher dietary sodium was associated with a greater ultrafiltration requirement, caloric and protein intake; sodium to calorie intake ratio was associated with a greater ultrafiltration requirement; and sodium to potassium ratio was associated with higher serum sodium. No indices were associated with the pre-dialysis systolic blood pressure. Cox regression modeling found that higher baseline dietary sodium and the ratio of sodium to calorie or potassium were each independently associated with greater all-cause mortality. No association between a prescribed dietary sodium restriction and mortality were found. Thus, higher reported dietary sodium intake is independently associated with greater mortality among prevalent hemodialysis patients. Randomized trials will be necessary to determine whether dietary sodium restriction improves survival.
Figures
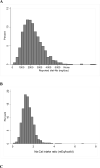
Figure 1
Distribution of daily dietary sodium intake (mg/day; Panel A), sodium:calorie intake ratio (mg/kcal/day; Panel B), sodium:potassium intake ratio (mg/mg/day; Panel C) and prescribed dietary sodium intake (mg/day; Panel D) at baseline.
Figure 1
Distribution of daily dietary sodium intake (mg/day; Panel A), sodium:calorie intake ratio (mg/kcal/day; Panel B), sodium:potassium intake ratio (mg/mg/day; Panel C) and prescribed dietary sodium intake (mg/day; Panel D) at baseline.
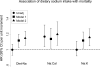
Figure 2
Hazard ratios (95% CIs) between measures of daily sodium intake and all-cause mortality. Sodium was considered as total intake (mg/day), sodium:calorie intake ratio (Na:Cal; mg/kcal/day) and sodium:potassium intake ratio (Na:K; mg/mg/day). Unadjusted estimates are indicated by circles. Estimates from Model 1 (squares) were adjusted for age, sex, race (black vs non-black), HEMO Study Kt/V and flux group assignments, post-dialysis weight, sex-by post-weight cross product terms, access (fistula, graft, catheter), congestive heart failure status (none, mild, moderate/severe), presence/ absence of diabetes and ischemic heart disease, urine volume (≤200mL/day, >200mL/day) and dialysis session length (≤180, 181-209, 210-239, ≥240min). Estimates from Model 2 (triangles) were additionally adjusted for serum sodium, albumin (≤3.5, 3.5-4.0 and >4.0 g/dL), phosphorus, creatinine and ultrafiltration requirement. All models were stratified on clinical center.
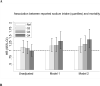
Figure 3
Hazard ratios (95% CIs) for all-cause mortality according to quartiles (Q; referent Q1) of reported daily dietary sodium intake (mg/day; Panel A), daily sodium:calorie intake ratio (mg/kcal/day; Panel B), and sodium:potassium intake ratio (mg/mg/day; Panel C). Estimates from Model 1 were adjusted for age, sex, race (black vs non-black), HEMO Study Kt/V and flux group assignments, post-dialysis weight, sex-by post-weight cross product terms, access (fistula, graft, catheter), congestive heart failure status (none, mild, moderate/severe), presence/ absence of diabetes and ischemic heart disease, urine volume (≤200mL/day, >200mL/day) and dialysis session length (≤180, 181-209, 210-239, ≥240min). Estimates from Model 2 were additionally adjusted for serum sodium, albumin (≤3.5, 3.5-4.0 and >4.0 g/dL), phosphorus, creatinine and ultrafiltration requirement. All models were stratified on clinical center.
Figure 3
Hazard ratios (95% CIs) for all-cause mortality according to quartiles (Q; referent Q1) of reported daily dietary sodium intake (mg/day; Panel A), daily sodium:calorie intake ratio (mg/kcal/day; Panel B), and sodium:potassium intake ratio (mg/mg/day; Panel C). Estimates from Model 1 were adjusted for age, sex, race (black vs non-black), HEMO Study Kt/V and flux group assignments, post-dialysis weight, sex-by post-weight cross product terms, access (fistula, graft, catheter), congestive heart failure status (none, mild, moderate/severe), presence/ absence of diabetes and ischemic heart disease, urine volume (≤200mL/day, >200mL/day) and dialysis session length (≤180, 181-209, 210-239, ≥240min). Estimates from Model 2 were additionally adjusted for serum sodium, albumin (≤3.5, 3.5-4.0 and >4.0 g/dL), phosphorus, creatinine and ultrafiltration requirement. All models were stratified on clinical center.
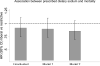
Figure 4
Associations between liberal (>2g/day) versus restrictive (≤2g/day; referent group; HR=1, not shown) prescribed sodium restriction and all-cause mortality. Estimates from Model 1 were adjusted for age, sex, race (black vs non-black), HEMO Study Kt/V and flux group assignments, post-dialysis weight, sex-by post-weight cross product terms, access (fistula, graft, catheter), congestive heart failure status (none, mild, moderate/severe), presence/ absence of diabetes and ischemic heart disease, urine volume (≤200mL/day, >200mL/day) and dialysis session length (≤180, 181-209, 210-239, ≥240min). Estimates from Model 2 were additionally adjusted for serum sodium, albumin (≤3.5, 3.5-4.0 and >4.0 g/dL), phosphorus, creatinine and ultrafiltration requirement. All models were stratified on clinical center.
Comment in
- Dietary sodium and clinical outcome in hemodialysis: where do we stand and what is next?
Rambod M, Tolouian R. Rambod M, et al. Kidney Int. 2012 Jul;82(2):130-2. doi: 10.1038/ki.2012.124. Kidney Int. 2012. PMID: 22743563
Similar articles
- Dietary sodium and clinical outcome in hemodialysis: where do we stand and what is next?
Rambod M, Tolouian R. Rambod M, et al. Kidney Int. 2012 Jul;82(2):130-2. doi: 10.1038/ki.2012.124. Kidney Int. 2012. PMID: 22743563 - Longitudinal Associations among Renal Urea Clearance-Corrected Normalized Protein Catabolic Rate, Serum Albumin, and Mortality in Patients on Hemodialysis.
Eriguchi R, Obi Y, Streja E, Tortorici AR, Rhee CM, Soohoo M, Kim T, Kovesdy CP, Kalantar-Zadeh K. Eriguchi R, et al. Clin J Am Soc Nephrol. 2017 Jul 7;12(7):1109-1117. doi: 10.2215/CJN.13141216. Epub 2017 May 10. Clin J Am Soc Nephrol. 2017. PMID: 28490436 Free PMC article. - Prescribed dietary phosphate restriction and survival among hemodialysis patients.
Lynch KE, Lynch R, Curhan GC, Brunelli SM. Lynch KE, et al. Clin J Am Soc Nephrol. 2011 Mar;6(3):620-9. doi: 10.2215/CJN.04620510. Epub 2010 Dec 9. Clin J Am Soc Nephrol. 2011. PMID: 21148246 Free PMC article. Clinical Trial. - Compared with usual sodium intake, low- and excessive-sodium diets are associated with increased mortality: a meta-analysis.
Graudal N, Jürgens G, Baslund B, Alderman MH. Graudal N, et al. Am J Hypertens. 2014 Sep;27(9):1129-37. doi: 10.1093/ajh/hpu028. Epub 2014 Mar 20. Am J Hypertens. 2014. PMID: 24651634 Review. - Impact of Salt Intake on the Pathogenesis and Treatment of Hypertension.
Rust P, Ekmekcioglu C. Rust P, et al. Adv Exp Med Biol. 2017;956:61-84. doi: 10.1007/5584_2016_147. Adv Exp Med Biol. 2017. PMID: 27757935 Review.
Cited by
- Management of hypertension and renin-angiotensin-aldosterone system blockade in adults with diabetic kidney disease: Association of British Clinical Diabetologists and the Renal Association UK guideline update 2021.
Banerjee D, Winocour P, Chowdhury TA, De P, Wahba M, Montero R, Fogarty D, Frankel AH, Karalliedde J, Mark PB, Patel DC, Pokrajac A, Sharif A, Zac-Varghese S, Bain S, Dasgupta I; Association of British Clinical Diabetologists and The Renal Association. Banerjee D, et al. BMC Nephrol. 2022 Jan 3;23(1):9. doi: 10.1186/s12882-021-02587-5. BMC Nephrol. 2022. PMID: 34979961 Free PMC article. - ISPD Cardiovascular and Metabolic Guidelines in Adult Peritoneal Dialysis Patients Part I - Assessment and Management of Various Cardiovascular Risk Factors.
Wang AY, Brimble KS, Brunier G, Holt SG, Jha V, Johnson DW, Kang SW, Kooman JP, Lambie M, McIntyre C, Mehrotra R, Pecoits-Filho R. Wang AY, et al. Perit Dial Int. 2015 Jul-Aug;35(4):379-87. doi: 10.3747/pdi.2014.00279. Perit Dial Int. 2015. PMID: 26228782 Free PMC article. Review. - Optimal dialysate sodium-what is the evidence?
Mc Causland FR, Waikar SS. Mc Causland FR, et al. Semin Dial. 2014 Mar;27(2):128-34. doi: 10.1111/sdi.12182. Epub 2014 Jan 23. Semin Dial. 2014. PMID: 24450281 Free PMC article. Review. - Dietary patterns and risk of death and progression to ESRD in individuals with CKD: a cohort study.
Gutiérrez OM, Muntner P, Rizk DV, McClellan WM, Warnock DG, Newby PK, Judd SE. Gutiérrez OM, et al. Am J Kidney Dis. 2014 Aug;64(2):204-13. doi: 10.1053/j.ajkd.2014.02.013. Epub 2014 Mar 27. Am J Kidney Dis. 2014. PMID: 24679894 Free PMC article. - Assessment and management of fluid overload in children on dialysis.
Hayes W, Paglialonga F. Hayes W, et al. Pediatr Nephrol. 2019 Feb;34(2):233-242. doi: 10.1007/s00467-018-3916-4. Epub 2018 Mar 9. Pediatr Nephrol. 2019. PMID: 29523958 Free PMC article. Review.
References
- Reinhardt HW, Seeliger E. Toward an Integrative Concept of Control of Total Body Sodium. News Physiol Sci. 2000;15:319–325. - PubMed
- Simpson FO. Sodium intake, body sodium, and sodium excretion. Lancet. 1988;2:25–29. - PubMed
- Kayikcioglu M, Tumuklu M, Ozkahya M, et al. The benefit of salt restriction in the treatment of end-stage renal disease by haemodialysis. Nephrol Dial Transplant. 2009;24:956–962. - PubMed
- Ozkahya M, Ok E, Toz H, et al. Long-term survival rates in haemodialysis patients treated with strict volume control. Nephrol Dial Transplant. 2006;21:3506–3513. - PubMed
- Maduell F, Navarro V. [Assessment of salt intake in hemodialysis]. Nefrologia. 2001;21:71–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK075941/DK/NIDDK NIH HHS/United States
- K23 DK075941/DK/NIDDK NIH HHS/United States
- K23 DK079056/DK/NIDDK NIH HHS/United States
- DK079056/DK/NIDDK NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical