Divergent role of the Hox gene Antennapedia in spiders is responsible for the convergent evolution of abdominal limb repression - PubMed (original) (raw)
Divergent role of the Hox gene Antennapedia in spiders is responsible for the convergent evolution of abdominal limb repression
Sara Khadjeh et al. Proc Natl Acad Sci U S A. 2012.
Abstract
Evolution often results in morphologically similar solutions in different organisms, a phenomenon known as convergence. However, there is little knowledge of the processes that lead to convergence at the genetic level. The genes of the Hox cluster control morphology in animals. They may also be central to the convergence of morphological traits, but whether morphological similarities also require similar changes in Hox gene function is disputed. In arthropods, body subdivision into a region with locomotory appendages ("thorax") and a region with reduced appendages ("abdomen") has evolved convergently in several groups, e.g., spiders and insects. In insects, legs develop in the expression domain of the Hox gene Antennapedia (Antp), whereas the Hox genes Ultrabithorax (Ubx) and abdominal-A mediate leg repression in the abdomen. Here, we show that, unlike Antp in insects, the Antp gene in the spider Achaearanea tepidariorum represses legs in the first segment of the abdomen (opisthosoma), and that Antp and Ubx are redundant in the following segment. The down-regulation of Antp in A. tepidariorum leads to a striking 10-legged phenotype. We present evidence from ectopic expression of the spider Antp gene in Drosophila embryos and imaginal tissue that this unique function of Antp is not due to changes in the Antp protein, but likely due to divergent evolution of cofactors, Hox collaborators or target genes in spiders and flies. Our results illustrate an interesting example of convergent evolution of abdominal leg repression in arthropods by altering the role of distinct Hox genes at different levels of their action.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Embryonic expression of the A. tepidariorum Antp gene. (A and B) At developmental stage 9, eight abdominal segments have formed (A, lateral view; B, ventral view). At-Antp is expressed in the opisthosoma with strongest expression in O1 (arrowhead in B). (C) Older embryo at late stage 11 in ventral view. The strong O1 expression domain of At-Antp becomes more prominent as expression levels in the remaining opisthosoma decrease. (D) Summary of the segmental expression domains of the Hox genes At-lab, At-Dfd, At-Scr, At-Antp, At-Ubx, and At-abd-A at stage 9. Note that the O1 segment only expresses At-Antp. ch, cheliceral segment; L1–L4, walking leg segments; O1–O8, opisthosomal segments; oc, ocular segment; pp, pedipalpal segment, wt, wild type.
Fig. 2.
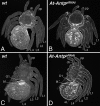
Ten-legged spiders result from Antp RNAi. (A and C) Wild-type larvae (A, dorsal view; C, ventral view). (B and D) Parental injection of At-Antp double-stranded RNA leads to the development of spiders with an extra pair of legs (B, dorsal view; D, ventral view) on opisthosomal segment O1. All other structures appear normal compared with the wild type; note also the normal book lungs on O2. bl, book lung; ch, chelicera; eL, ectopic leg; L, walking leg; O, opisthosomal segment; pp, pedipalp.
Fig. 3.
The additional legs are genuine walking legs. Expression of leg marker genes in At-Antp RNAi embryos at late stage 11 (shortly before dorsal closure). (A) At-Dll is expressed in the distal portion of the ectopic legs on O1 similar to the walking legs. (B) Expression of At-dac in the ectopic legs shows the usual medial expression as in the normal legs. (C) At-exd-1 is expressed in a proximal domain in the ectopic legs and in L1–L4, including the medial ring of expression (albeit faint) (star). (D) At-hth-1 is expressed throughout the entire leg apart from the tip in the legs and in the ectopic legs. (E) At-Dfd expression is similar in the ectopic legs and the normal legs. Note that expression in L1 and L2 reaches further proximal than in L3 and L4, where expression is restricted to the tip. (F) At-Scr marks each of the legs with a unique pattern. The expression pattern in the ectopic legs differs from the patterns of the normal legs and shows faint distal rings and a strong expression domain in the tip (arrowhead). bl, book lung; ch, chelicera; eL, ectopic leg; L, walking leg; O, opisthosomal segment; pp, pedipalp.
Fig. 4.
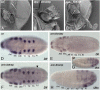
Double RNAi with At-Antp and At-Ubx. (A) At-AntpUbx larva in ventral view. Note the ectopic leg (eL) on O1 and the additional appendage rudiment (ar) on O2. (B and C) Expression of At-Scr (B) and At-Dfd (C) in the O2 appendage rudiment at late stage 11. (B) Preparation of the opisthosomal appendages (lateral view, anterior to the left) to show the expression of Scr in the proximal O2 rudiment; Scr is not expressed in the distal outgrowth of the O2 limb (arrowhead) and the tracheal bud (tr). (C) Ventral view. Dfd is expressed in the legs, and the eL on O1 and the O2 rudiment. (D_–_F) Expression of Dll at late stage 11. (D) Ventral view of At-AntpUbx RNAi embryo; note Dll expression in the O2 rudiment. (E and F) Comparison of Dll expression in the opisthosoma of wild-type (E) and At-AntpUbx RNAi embryos (F). Lateral view, anterior to the left. Note that in the wild type, the book lung buds on O2 do not express Dll, whereas Dll is expressed in the distal outgrowth of the O2 appendage rudiment (arrowhead). as, anterior spinneret bud; ch, chelicera; L, walking leg; pp, pedipalp; ps, posterior spinneret bud; tr, trachea bud.
Fig. 5.
Misexpression of At-Antp in Drosophila melanogaster. (A_–_C) Misexpression of At-Antp in the antenna imaginal disk leads to arista-to-tarsus transformation. (A) The wildtype antenna consists of 3 antennal segments and the arista. (C) Misexpression of At-Antp using the _dpp_-Gal4 driver transforms the arista into tarsal identity, similar to the phenotype of Dm-Antp misexpression (B). (D_–_H) Ubiquitous expression of At-Antp in Drosophila melanogaster embryos does not repress Dll expression but leads to ectopic Dll expression in the first abdominal segment. (D) In wild-type stage 11 embryos, Dll is expressed in the thoracic leg primordia and in antennal, maxillary and labial segments and in the primordium of the labrum. (E) _arm_-Gal4 driven ubiquitous misexpression of Dm-Ubx leads to repression of Dll in the thorax. (F) _arm_-Gal4 driven ubiquitous misexpression of At-Antp shows no repression of Dll, but extra patches of Dll expression appear in the A1 segment (arrowhead). (G and H) Embryos misexpressing At-Antp (H) show normal expression of Dm-Ubx, compared with the wild type (G). This indicates that the derepression of Dll in A1 in arm:At-Antp embryos is not due to At-Antp mediated repression of Ubx. A1, first abdominal segment; Ant, antennal segment; Lab, labial segment; Mx, maxillary segment; T, thoracic segment.
Similar articles
- Candidate gene screen for potential interaction partners and regulatory targets of the Hox gene labial in the spider Parasteatoda tepidariorum.
Schomburg C, Turetzek N, Prpic NM. Schomburg C, et al. Dev Genes Evol. 2020 Mar;230(2):105-120. doi: 10.1007/s00427-020-00656-7. Epub 2020 Feb 8. Dev Genes Evol. 2020. PMID: 32036446 Free PMC article. - Regressive evolution of the arthropod tritocerebral segment linked to functional divergence of the Hox gene labial.
Pechmann M, Schwager EE, Turetzek N, Prpic NM. Pechmann M, et al. Proc Biol Sci. 2015 Sep 7;282(1814):20151162. doi: 10.1098/rspb.2015.1162. Proc Biol Sci. 2015. PMID: 26311666 Free PMC article. - Specificity of Distalless repression and limb primordia development by abdominal Hox proteins.
Gebelein B, Culi J, Ryoo HD, Zhang W, Mann RS. Gebelein B, et al. Dev Cell. 2002 Oct;3(4):487-98. doi: 10.1016/s1534-5807(02)00257-5. Dev Cell. 2002. PMID: 12408801 - Hox genes and the evolution of the arthropod body plan.
Hughes CL, Kaufman TC. Hughes CL, et al. Evol Dev. 2002 Nov-Dec;4(6):459-99. doi: 10.1046/j.1525-142x.2002.02034.x. Evol Dev. 2002. PMID: 12492146 Review. - Patterning mechanisms and morphological diversity of spider appendages and their importance for spider evolution.
Pechmann M, Khadjeh S, Sprenger F, Prpic NM. Pechmann M, et al. Arthropod Struct Dev. 2010 Nov;39(6):453-67. doi: 10.1016/j.asd.2010.07.007. Arthropod Struct Dev. 2010. PMID: 20696272 Review.
Cited by
- A Novel Expression Domain of extradenticle Underlies the Evolutionary Developmental Origin of the Chelicerate Patella.
Klementz BC, Brenneis G, Hinne IA, Laumer EM, Neu SM, Hareid GM, Gainett G, Setton EVW, Simian C, Vrech DE, Joyce I, Barnett AA, Patel NH, Harvey MS, Peretti AV, Gulia-Nuss M, Sharma PP. Klementz BC, et al. Mol Biol Evol. 2024 Sep 4;41(9):msae188. doi: 10.1093/molbev/msae188. Mol Biol Evol. 2024. PMID: 39235104 Free PMC article. - Posterior Hox gene reduction in an arthropod: Ultrabithorax and Abdominal-B are expressed in a single segment in the mite Archegozetes longisetosus.
Barnett AA, Thomas RH. Barnett AA, et al. Evodevo. 2013 Aug 30;4(1):23. doi: 10.1186/2041-9139-4-23. Evodevo. 2013. PMID: 23991696 Free PMC article. - Rapid diversification of homothorax expression patterns after gene duplication in spiders.
Turetzek N, Khadjeh S, Schomburg C, Prpic NM. Turetzek N, et al. BMC Evol Biol. 2017 Jul 14;17(1):168. doi: 10.1186/s12862-017-1013-0. BMC Evol Biol. 2017. PMID: 28709396 Free PMC article. - Extreme multisegmentation in a giant bivalved arthropod from the Cambrian Burgess Shale.
Izquierdo-López A, Caron JB. Izquierdo-López A, et al. iScience. 2022 Jun 25;25(7):104675. doi: 10.1016/j.isci.2022.104675. eCollection 2022 Jul 15. iScience. 2022. PMID: 35845166 Free PMC article.
References
- Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276:565–570. - PubMed
- Struhl G. A homoeotic mutation transforming leg to antenna in Drosophila. Nature. 1981;292:635–638. - PubMed
- Regulski M, et al. Homeo box genes of the Antennapedia and bithorax complexes of Drosophila. Cell. 1985;43:71–80. - PubMed
- Akam M, Dawson I, Tear G. Homeotic genes and the control of segment diversity. Development. 1988;104:123–133.
- Carroll SB. Homeotic genes and the evolution of arthropods and chordates. Nature. 1995;376:479–485. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources