Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma - PubMed (original) (raw)
Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma
Shannon L Russell et al. EMBO Rep. 2012.
Abstract
Allergic asthma rates have increased steadily in developed countries, arguing for an environmental aetiology. To assess the influence of gut microbiota on experimental murine allergic asthma, we treated neonatal mice with clinical doses of two widely used antibiotics--streptomycin and vancomycin--and evaluated resulting shifts in resident flora and subsequent susceptibility to allergic asthma. Streptomycin treatment had little effect on the microbiota and on disease, whereas vancomycin reduced microbial diversity, shifted the composition of the bacterial population and enhanced disease severity. Neither antibiotic had a significant effect when administered to adult mice. Consistent with the 'hygiene hypothesis', our data support a neonatal, microbiota-driven, specific increase in susceptibility to experimental murine allergic asthma.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
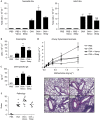
Neonatal vancomycin treatment exacerbates allergic asthma. (A) Total cellular infiltrates from bronchoalveolar lavage (BAL) of control or antibiotic-treated mice challenged with ovalbumin (OVA) or PBS. (B) Eosinophil numbers in the BAL quantified by cytospin. (C) Serum OVA-specific IgE responses measured by enzyme-linked immunosorbent assay. (D) Airway hyperresponsiveness, measured by changes in resistance (R) in response to increasing doses of methacholine administered intravenously. (E) Total pathological scores and representative haematoxylin- and eosin-stained lung sections. Scale bar, 300 μm. All assessments were made on day 26. The data are shown as means of 5–7 mice per group±s.e.m. and represent at least two independent experiments. Statistics shown are based on comparisons to OVA-challenged controls. Antibiotic-treated animals in B–E were treated neonatally. *P<0.05, **P<0.01; Abx, antibiotics; ND, none detected; NS, not significant; Strep, streptomycin; Vanco, vancomycin.
Figure 2
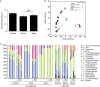
Antibiotic treatment alters gut bacterial communities. (A) Total bacteria in faeces of control or antibiotic-treated mice quantitated by SYBR green. These data are shown as means of 3–5 mice per group±s.e.m. and represent two independent experiments. *P<0.05 relative to control; NS, not significant. (B) Bacterial communities from ileum and faeces of naive or ovalbumin (OVA)-challenged control and antibiotic-treated mice were compared using principal coordinate analysis (PCO). (C) Family level phylogenetic classification of 16S rRNA gene frequencies in faeces collected from naive or OVA-challenged control and antibiotic-treated animals. Those indicated with a classification level other than family level (f) could only be identified confidently to the level indicated. Classification scheme: k, kingdom; p, phylum; c, class; o, order; f, family. Each bar represents one mouse. Rare taxa were removed from the legend, but still included in the graph. Antibiotic-treated animals in A and B were treated neonatally. Abx, antibiotics; Strep, streptomycin; Vanco, vancomycin.
Figure 3
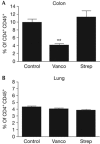
Vancomycin treatment reduces CD4+CD25+Foxp3+ regulatory T-cell accumulation in the colon but not in the lung. At 7 weeks of age, the percentage of CD25+Foxp3+ cells within the CD45+CD4+ cell population in the colon (A) or lung (B) of naive mice treated with neonatal antibiotics was analysed. The data shown are means of three mice per group±s.e.m. and represent three independent experiments. Vanco, vancomycin; Strep, streptomycin. **P<0.01.
Comment in
- Mucosal immunology: the good the gut bugs do.
Leavy O. Leavy O. Nat Rev Immunol. 2012 Apr 13;12(5):319. doi: 10.1038/nri3213. Nat Rev Immunol. 2012. PMID: 22498779 No abstract available.
Similar articles
- Perinatal antibiotic treatment affects murine microbiota, immune responses and allergic asthma.
Russell SL, Gold MJ, Willing BP, Thorson L, McNagny KM, Finlay BB. Russell SL, et al. Gut Microbes. 2013 Mar-Apr;4(2):158-64. doi: 10.4161/gmic.23567. Epub 2013 Jan 18. Gut Microbes. 2013. PMID: 23333861 Free PMC article. Review. - Perinatal antibiotic-induced shifts in gut microbiota have differential effects on inflammatory lung diseases.
Russell SL, Gold MJ, Reynolds LA, Willing BP, Dimitriu P, Thorson L, Redpath SA, Perona-Wright G, Blanchet MR, Mohn WW, Finlay BB, McNagny KM. Russell SL, et al. J Allergy Clin Immunol. 2015 Jan;135(1):100-9. doi: 10.1016/j.jaci.2014.06.027. Epub 2014 Aug 18. J Allergy Clin Immunol. 2015. PMID: 25145536 - Early-life vancomycin treatment promotes airway inflammation and impairs microbiome homeostasis.
Yang X, Feng H, Zhan X, Zhang C, Cui R, Zhong L, Ying S, Chen Z. Yang X, et al. Aging (Albany NY). 2019 Apr 13;11(7):2071-2081. doi: 10.18632/aging.101901. Aging (Albany NY). 2019. PMID: 30981206 Free PMC article. - Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection.
Sekirov I, Tam NM, Jogova M, Robertson ML, Li Y, Lupp C, Finlay BB. Sekirov I, et al. Infect Immun. 2008 Oct;76(10):4726-36. doi: 10.1128/IAI.00319-08. Epub 2008 Aug 4. Infect Immun. 2008. PMID: 18678663 Free PMC article. - Role of microbiome in the pathophysiology and disease course of asthma.
Singanayagam A, Ritchie AI, Johnston SL. Singanayagam A, et al. Curr Opin Pulm Med. 2017 Jan;23(1):41-47. doi: 10.1097/MCP.0000000000000333. Curr Opin Pulm Med. 2017. PMID: 27755161 Review.
Cited by
- Correlations of Host Genetics and Gut Microbiome Composition.
Dąbrowska K, Witkiewicz W. Dąbrowska K, et al. Front Microbiol. 2016 Aug 30;7:1357. doi: 10.3389/fmicb.2016.01357. eCollection 2016. Front Microbiol. 2016. PMID: 27625642 Free PMC article. Review. - Mechanisms of intestinal dysbiosis: new insights into tuft cell functions.
Coutry N, Gasmi I, Herbert F, Jay P. Coutry N, et al. Gut Microbes. 2024 Jan-Dec;16(1):2379624. doi: 10.1080/19490976.2024.2379624. Epub 2024 Jul 23. Gut Microbes. 2024. PMID: 39042424 Free PMC article. Review. - Gut microbiota and immunity relevance in eubiosis and dysbiosis.
Al-Rashidi HE. Al-Rashidi HE. Saudi J Biol Sci. 2022 Mar;29(3):1628-1643. doi: 10.1016/j.sjbs.2021.10.068. Epub 2021 Oct 30. Saudi J Biol Sci. 2022. PMID: 35280528 Free PMC article. Review. - Dysbiosis of the respiratory tract mucosa-how microbial imbalances lead to asthma.
Peebles AB, Peebles RS Jr. Peebles AB, et al. Ann Transl Med. 2023 Jan 15;11(1):1. doi: 10.21037/atm-22-6009. Epub 2022 Dec 13. Ann Transl Med. 2023. PMID: 36760263 Free PMC article. No abstract available. - Dual Effect of Low-Molecular-Weight Bioregulators of Bacterial Origin in Experimental Model of Asthma.
Guryanova SV, Gigani OB, Gudima GO, Kataeva AM, Kolesnikova NV. Guryanova SV, et al. Life (Basel). 2022 Jan 27;12(2):192. doi: 10.3390/life12020192. Life (Basel). 2022. PMID: 35207480 Free PMC article.
References
- Lai CK, Beasley R, Crane J, Foliaki S, Shah J, Weiland S (2009) Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 64: 476–483 - PubMed
- Penders J, Stobberingh EE, van den Brandt PA, Thijs C (2007) The role of the intestinal microbiota in the development of atopic disorders. Allergy 62: 1223–1236 - PubMed
- Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrlander C, Heederik D, Piarroux R, von Mutius E (2011) Exposure to environmental microorganisms and childhood asthma. N Engl J Med 364: 701–709 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical