Converting cancer therapies into cures: lessons from infectious diseases - PubMed (original) (raw)
Converting cancer therapies into cures: lessons from infectious diseases
Michael S Glickman et al. Cell. 2012.
Abstract
During the past decade, cancer drug development has shifted from a focus on cytotoxic chemotherapies to drugs that target specific molecular alterations in tumors. Although these drugs dramatically shrink tumors, the responses are temporary. Research is now focused on overcoming drug resistance, a frequent cause of treatment failure. Here we reflect on analogous challenges faced by researchers in infectious diseases. We compare and contrast the resistance mechanisms arising in cancer and infectious diseases and discuss how approaches for overcoming viral and bacterial infections, such as HIV and tuberculosis, are instructive for developing a more rational approach for cancer therapy. In particular, maximizing the effect of the initial treatment response, which often requires synergistic combination therapy, is foremost among these approaches. A remaining challenge in both fields is identifying drugs that eliminate drug-tolerant "persister" cells (infectious disease) or tumor-initiating/stem cells (cancer) to prevent late relapse and shorten treatment duration.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Figure 1. Mechanisms of Resistance to Antimicrobials and Targeted Anticancer Agents
(A) Resistance via target mutation. This mechanism has been well described in both antimicrobial resistance and tumor cell resistance. The red drug binds tightly to its target (black square). A mutational event leads to alteration in the binding site for the drug (yellow circle), leading to loss of drug binding. This mechanism governs resistance to β-lactam antimicrobials (and other antimicrobial classes) as well as resistance to kinase inhibitors. (B) Resistance via bypass pathways. Treatment with antimicrobial or anticancer agents (red lines) leads to a block in the pathway converting X to Y. Conceptually, Y can be a metabolite or a phenotypic state (e.g., cell proliferation). Resistance to the effect of the drug can be mediated by upregulation of a parallel pathway that allows Y to be restored. This mechanism of resistance has been documented in anticancer therapy, for example, amplification of MET to bypass a drug-induced block in EGFR signaling. (C) Resistance by drug destruction or modification. Bacterial enzymes, such as β-lactamases or aminoglycoside-modifying enzymes, mediate antimicrobial resistance by drug destruction or modification. This mechanism has not been described for resistance to anticancer agents, although modification of anticancer agents through CYPs can affect efficacy (although this effect is not mediated by the tumor cell). (D) Intrinsic, nonmutational, resistance. Here the population of cells (either tumor or microbial) are genotypically identical. The red cells are drug sensitive and are rapidly killed by antimicrobial or anticancer therapy, but the yellow cells are poorly killed by antimicrobial or cancer therapy (tolerant). These tolerant cells are called microbial persisters or cancer stem cells. Therapy with antimicrobials or anticancer agents leads to substantial killing, but the persister cells are able to resist treatment and can repopulate the infection or tumor with drug-sensitive cells, causing disease relapse.
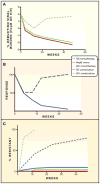
Figure 2. Relative Efficacy of Mono- versus Combination Therapy for Three Chronic Infections and Effect on Resistance
(A) The three curves schematically indicate the reduction in viral burden during monotherapy of HIV (dashed green line), combination therapy for HIV (solid green line), and monotherapy for hepatitis B (solid red line). Monotherapy of HIV produces a transient reduction in viral load, which becomes more dramatic and sustained with combination therapy. In contrast, monotherapy for hepatitis B (with entecavir or tenofovir) produces sustained virologic suppression. See text for specific references. (B) Monotherapy and combination therapy for tuberculosis. The y axis schematically represents clinical response (reduction in bacterial load, clinical improvement, radiographic improvement). Monotherapy and combination therapy have similar efficacy early in treatment, but the benefit of monotherapy is not sustained. (C) Effect of combination therapy on emergence of resistance. The y axis represents the % of patients with resistant bacteria or viruses according to week of treatment. HIV and TB resistance emerges rapidly during mono-therapy, leading to the loss of therapeutic effect depicted in panel A (HIV) and panel B (TB). Combination therapy for HIV and TB suppresses the emergence of resistant organisms and allows the sustained therapeutic benefit depicted in (A) and (B). In contrast, monotherapy for hepatitis B with entecavir or tenofovir is not associated with the emergence of resistant viruses, allowing sustained therapeutic benefit with monotherapy.
Similar articles
- Managing drug resistance in cancer: lessons from HIV therapy.
Bock C, Lengauer T. Bock C, et al. Nat Rev Cancer. 2012 Jun 7;12(7):494-501. doi: 10.1038/nrc3297. Nat Rev Cancer. 2012. PMID: 22673150 Review. - Improvement of conventional anti-cancer drugs as new tools against multidrug resistant tumors.
Dallavalle S, Dobričić V, Lazzarato L, Gazzano E, Machuqueiro M, Pajeva I, Tsakovska I, Zidar N, Fruttero R. Dallavalle S, et al. Drug Resist Updat. 2020 May;50:100682. doi: 10.1016/j.drup.2020.100682. Epub 2020 Feb 7. Drug Resist Updat. 2020. PMID: 32087558 - [Development of antituberculous drugs: current status and future prospects].
Tomioka H, Namba K. Tomioka H, et al. Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese. - New tools for old drugs: Functional genetic screens to optimize current chemotherapy.
Gerhards NM, Rottenberg S. Gerhards NM, et al. Drug Resist Updat. 2018 Jan;36:30-46. doi: 10.1016/j.drup.2018.01.001. Epub 2018 Jan 12. Drug Resist Updat. 2018. PMID: 29499836 Free PMC article. Review. - Mechanisms of chemotherapeutic drug resistance in cancer therapy--a quick review.
Liu FS. Liu FS. Taiwan J Obstet Gynecol. 2009 Sep;48(3):239-44. doi: 10.1016/S1028-4559(09)60296-5. Taiwan J Obstet Gynecol. 2009. PMID: 19797012 Review.
Cited by
- Adaptation or selection--mechanisms of castration-resistant prostate cancer.
Zong Y, Goldstein AS. Zong Y, et al. Nat Rev Urol. 2013 Feb;10(2):90-8. doi: 10.1038/nrurol.2012.237. Epub 2012 Dec 18. Nat Rev Urol. 2013. PMID: 23247694 Review. - Tumour evolution in hepatocellular carcinoma.
Craig AJ, von Felden J, Garcia-Lezana T, Sarcognato S, Villanueva A. Craig AJ, et al. Nat Rev Gastroenterol Hepatol. 2020 Mar;17(3):139-152. doi: 10.1038/s41575-019-0229-4. Epub 2019 Dec 2. Nat Rev Gastroenterol Hepatol. 2020. PMID: 31792430 Review. - Addiction to multiple oncogenes can be exploited to prevent the emergence of therapeutic resistance.
Choi PS, Li Y, Felsher DW. Choi PS, et al. Proc Natl Acad Sci U S A. 2014 Aug 12;111(32):E3316-24. doi: 10.1073/pnas.1406123111. Epub 2014 Jul 28. Proc Natl Acad Sci U S A. 2014. PMID: 25071175 Free PMC article. - BRAF inhibitors in clinical oncology.
Morris V, Kopetz S. Morris V, et al. F1000Prime Rep. 2013 Apr 2;5:11. doi: 10.12703/P5-11. Print 2013. F1000Prime Rep. 2013. PMID: 23585929 Free PMC article. - Emerging Cellular Therapies for Cancer.
Guedan S, Ruella M, June CH. Guedan S, et al. Annu Rev Immunol. 2019 Apr 26;37:145-171. doi: 10.1146/annurev-immunol-042718-041407. Epub 2018 Dec 10. Annu Rev Immunol. 2019. PMID: 30526160 Free PMC article. Review.
References
- Allan EJ, Hoischen C, Gumpert J. Bacterial L-forms. Adv Appl Microbiol. 2009;68:1–39. - PubMed
- Altamirano M, Marostenmaki J, Wong A, FitzGerald M, Black WA, Smith JA. Mutations in the catalase-peroxidase gene from isoniazid-resistant Mycobacterium tuberculosis isolates. J Infect Dis. 1994;169:1162–1165. - PubMed
- Antonescu CR, Besmer P, Guo T, Arkun K, Hom G, Koryotowski B, Leversha MA, Jeffrey PD, Desantis D, Singer S, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res. 2005;11:4182–4190. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous