Direct reprogramming of human astrocytes into neural stem cells and neurons - PubMed (original) (raw)
. 2012 Aug 1;318(13):1528-41.
doi: 10.1016/j.yexcr.2012.02.040. Epub 2012 Mar 8.
Monica Nizzardo, Chiara Simone, Marianna Falcone, Chiara Donadoni, Sabrina Salani, Federica Rizzo, Martina Nardini, Giulietta Riboldi, Francesca Magri, Chiara Zanetta, Irene Faravelli, Nereo Bresolin, Giacomo P Comi
Affiliations
- PMID: 22426197
- PMCID: PMC3405531
- DOI: 10.1016/j.yexcr.2012.02.040
Direct reprogramming of human astrocytes into neural stem cells and neurons
Stefania Corti et al. Exp Cell Res. 2012.
Abstract
Generating neural stem cells and neurons from reprogrammed human astrocytes is a potential strategy for neurological repair. Here we show dedifferentiation of human cortical astrocytes into the neural stem/progenitor phenotype to obtain progenitor and mature cells with a neural fate. Ectopic expression of the reprogramming factors OCT4, SOX2, or NANOG into astrocytes in specific cytokine/culture conditions activated the neural stem gene program and induced generation of cells expressing neural stem/precursor markers. Pure CD44+ mature astrocytes also exhibited this lineage commitment change and did not require passing through a pluripotent state. These astrocyte-derived neural stem cells gave rise to neurons, astrocytes, and oligodendrocytes and showed in vivo engraftment properties. ASCL1 expression further promoted neuronal phenotype acquisition in vitro and in vivo. Methylation analysis showed that epigenetic modifications underlie this process. The restoration of multipotency from human astrocytes has potential in cellular reprogramming of endogenous central nervous system cells in neurological disorders.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Fig. 1
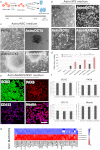
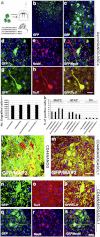
OCT4/SOX2/NANOG-transduced human cortical astrocytes give rise to neural stem cell (NSC) colonies. a) Schematic illustration of NSC generation from human cortical astrocytes (Astro). Human astrocytes were transfected with OCT4, SOX2, or NANOG. The cells were cultured in ESC/iPSC or NSC media. NSC colonies were observed between days 14 and 21 post-transfection. b, c) Bright-field images of human untransduced astrocytes (Astro) and OCT4- (AstroOCT4), SOX2- (AstroSOX2), or NANOG- (AstroNANOG) transduced astrocyte colonies (dashed lines indicate the spheres) in ESC/iPSC medium or NSC medium (n = 12/condition). Scale bar b: 150 μm; scale bar c, Astro and AstroOCT4: 150 μm; AstroSOX2: 75 μm; AstroNANOG: 100 μm. d) Quantification of colonies in human astrocytes after transduction in ESC/iPSC medium (gray bar) or in neuronal medium (pink bar) at 21 days (12 biological replicates; error bars, s.d.; *P < .00001). e) Immunocytochemical expression of SOX2, PAX6, CD133, and nestin in AstroNANOG colonies. Similar results were obtained with AstroOCT4 and AstroSOX2 colonies. Scale bar: 150 μm. f) Quantification of NSC marker expression as a percentage of positive cells in AstroOCT4, AstroSOX2, and AstroNANOG colonies. g) Global gene analysis of NSC marker expression of Astro vs AstroNANOG cells. For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.
Fig. 2
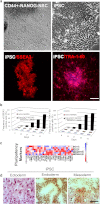
Conversion of astrocytes into NSCs by OCT4/SOX2 and NANOG does not require the pluripotent state. a) A colony of CD44 + NANOG-NSCs presented a different morphological aspect under contrast-phase microscopic observation relative to the iPSC colony obtained from fibroblasts by contemporaneous transfection of OCT4/SOX2/NANOG/LIN28. iPSCs were positive for pluripotency markers SSEA3 and TRA-1-60. Scale bar: 150 μm. b) Quantitative analysis of SSEA3 and Tra-1-60 in Astro, AstroNANOG, and human fibroblasts transduced with OCT4, SOX2, NANOG, and LIN28, over the human iPSC derivation timeline. c) Pluripotency gene profile in parental astrocytes and in AstroNANOG. d) Teratomas derived from human iPSCs and testicular sections from mice injected with astrocytes, AstroNANOG, and saline (CTR). Scale bar: 300 μm.
Fig. 3
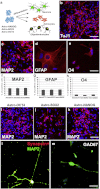
In vitro generation of three neuroectodermal lineages in particular in neurons from AstroOCT4/SOX2 or NANOG. Protocol of AstroNSC differentiation into neurons, astrocytes, and oligodendrocytes. AstroNSCs were cultured in human neuronal differentiation medium. The cell phenotype was determined after 15 days of differentiation. b–e) Differentiation of AstroNANOG cells into neurons positive for TuJ1 (b) and MAP2 (c), astrocytes positive for GFAP (d), and oligodendrocytes positive for O4 (e). f–h) Quantification of the differentiation efficiency into neurons (MAP2), astrocytes (GFAP), and O4 for the three conditions (NANOG, OCT4, SOX2). i–k) AstroOCT4/SOX2/NANOG had similar neuronal differentiation abilities as demonstrated here with immunocytochemistry for MAP2. l–m) The AstroNSC-derived neurons acquired a complex mature phenotype and were positive for synapsin (l, shown here in red as double staining with MAP2, green) and neurotransmitters like GAD67 positivity, enzyme for the biosynthesis of the neurotransmitter GABA suggests. Scale bar: b, c, i, j, k: 150 μm; d, e: 50 μm; l, m: 50 μm.
Fig. 4
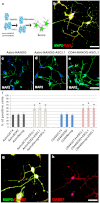
ASCL1 promotes neuronal differentiation of Astro and CD44 + NANOG cells. a) Schematic illustration of Astro and CD44 + NANOG-ASCL1 differentiation into neurons. Astro/CD44 + NSCs were cultured in human neuronal differentiation medium. The cell phenotype was analyzed after 15 days. b) AstroNANOG-ASCL neurons presented long dendritic arborization and axons and were positive for pan-neuronal markers MAP2 (green) and TuJ1 (red), shown here as merged signal. c–e) Neurons differentiated from AstroNANOG-ASCL and CD44-NANOG-ASCL1 were present at higher rates and with a complex mature morphology in culture relative to AstroNANOG. The neurons are shown here with immunocytochemistry for MAP2. f) Quantification of the differentiation efficiency into neurons (MAP2), demonstrating that efficiency was significantly higher with AstroNANOG-ASCL1 and CD44-NANOG-ASCL1 vs AstroCD44-NANOG. g, h) The AstroNSC-derived neurons were positive for GAD67 (shown here in red as double staining with MAP2, green (g) or in single staining (h)). Scale bar: b: 50 μm; c, d: 150 μm; g: 50 μm; h: 70 μm.
Fig. 5
AstroNSCs transplanted in rodent brain survive and generate neurons. a) For in vivo experiments, we transplanted AstroNSCs (AstroNANOG, CD44NANOG ± ASCL1) intracerebroventricularly in the mouse brain. To trace the fate of transplanted cells, cells were modified to express the gene reporter GFP. b, c) After transplantation of AstroNANOG cells, we demonstrated the presence of integrated donor cells in brain cortical areas. d–i) A significant proportion of GFP cells (d, g: green) acquired a neuronal phenotype as demonstrated here by the expression of the neuronal markers NeuN (e: red) and TuJ1 (h: red). f, i: merged images. j, k) Quantification of donor-engrafted cell number (j) and of different phenotypes acquired (k, expressed as percentage of positive cells: neurons (MAP2), astrocytes (GFAP), and oligodendrocytes (O4)), for each condition. l, m) Transplantation of CD44-NANOG-ASCL1 cells gave rise to a higher proportion of mature neurons relative to the CD44-NANOG population as shown here by immunohistochemistry for MAP2 (red) merged (yellow) with GFP (green). n–s) The neurons derived from CD44-NANOG-ASCL1-GFP cells (n, q) presented a complex phenotype and were positive for neuronal markers like TuJ1 (o: red) and NeuN (r: red). p, s: merged images. Scale bar: b: 300 μm; c: 80 μm; d–i: 75 μm; l, m: 75 μm; n–p: 50 μm; q–s: 80 μm.
Fig. 6
Epigenetic and gene expression modifications associated with astrocyte reprogramming. a) We compared the methylation pattern of three human AstroNANOG-NSC clones to the astrocytes from which they were derived. DNA Methylation Find-Peaks analysis identified regions of significant positive enrichment in microarray data as shown here in the case of SOX1 as an example. b) A total of 6667 of 67,652 regions analyzed were found to significantly differ. The number of differentially methylated regions, R-DMRs, that were hypermethylated in astrocytes significantly predominated over hypomethylated. This diagram shows the log2 values of the fold changes (Astro/NANOG). The distribution shows a clear majority of positive values, i.e., the respective fold changes were mostly > 1. c, d) We then performed a gene ontology (GO) annotation analysis of the genes near these regions (within 1000 bp of the transcriptional start site (TSS) of a gene). The genes that were hypermethylated in astrocytes compared to AstroNSCs are shown in c, while the genes that were hypomethylated in astrocytes compared to AstroNANOG are shown in d. e–j: Correlations for the global gene expression profile of astrocytes and AstroNANOG and the OCT4, SOX2, and NANOG occupancy. e) Relative expression of genes occupied by OCT4, SOX2, and NANOG and their heat map representation (f) as well as relative intensity (g). Binding site sequences of OCT4, SOX2, and NANOG (h). Relative expression of genes occupied by OCT, SOX2, or NANOG, individually considered, and their relative intensity.
Similar articles
- Sonic Hedgehog Effectively Improves Oct4-Mediated Reprogramming of Astrocytes into Neural Stem Cells.
Yang H, Liu C, Fan H, Chen B, Huang D, Zhang L, Zhang Q, An J, Zhao J, Wang Y, Hao D. Yang H, et al. Mol Ther. 2019 Aug 7;27(8):1467-1482. doi: 10.1016/j.ymthe.2019.05.006. Epub 2019 May 16. Mol Ther. 2019. PMID: 31153826 Free PMC article. - Human induced pluripotent stem cell-derived neural stem cells survive, migrate, differentiate, and improve neurologic function in a rat model of middle cerebral artery occlusion.
Yuan T, Liao W, Feng NH, Lou YL, Niu X, Zhang AJ, Wang Y, Deng ZF. Yuan T, et al. Stem Cell Res Ther. 2013 Jun 14;4(3):73. doi: 10.1186/scrt224. Stem Cell Res Ther. 2013. PMID: 23769173 Free PMC article. - Alternative Routes to Induced Pluripotent Stem Cells Revealed by Reprogramming of the Neural Lineage.
Jackson SA, Olufs ZP, Tran KA, Zaidan NZ, Sridharan R. Jackson SA, et al. Stem Cell Reports. 2016 Mar 8;6(3):302-11. doi: 10.1016/j.stemcr.2016.01.009. Epub 2016 Feb 18. Stem Cell Reports. 2016. PMID: 26905202 Free PMC article. - Chemically Induced Reprogramming of Somatic Cells to Pluripotent Stem Cells and Neural Cells.
Biswas D, Jiang P. Biswas D, et al. Int J Mol Sci. 2016 Feb 6;17(2):226. doi: 10.3390/ijms17020226. Int J Mol Sci. 2016. PMID: 26861316 Free PMC article. Review. - [Hypoxia epigenetically bestows astrocytic differentiation potential on human pluripotent cell-derived neural stem/precursor cells].
Yasui T, Nakashima K. Yasui T, et al. Nihon Yakurigaku Zasshi. 2019;153(2):54-60. doi: 10.1254/fpj.153.54. Nihon Yakurigaku Zasshi. 2019. PMID: 30745514 Review. Japanese.
Cited by
- Stem cells for spinal cord injury: Strategies to inform differentiation and transplantation.
Iyer NR, Wilems TS, Sakiyama-Elbert SE. Iyer NR, et al. Biotechnol Bioeng. 2017 Feb;114(2):245-259. doi: 10.1002/bit.26074. Epub 2016 Sep 21. Biotechnol Bioeng. 2017. PMID: 27531038 Free PMC article. Review. - SOX2+ cell population from normal human brain white matter is able to generate mature oligodendrocytes.
Oliver-De La Cruz J, Carrión-Navarro J, García-Romero N, Gutiérrez-Martín A, Lázaro-Ibáñez E, Escobedo-Lucea C, Perona R, Belda-Iniesta C, Ayuso-Sacido A. Oliver-De La Cruz J, et al. PLoS One. 2014 Jun 5;9(6):e99253. doi: 10.1371/journal.pone.0099253. eCollection 2014. PLoS One. 2014. PMID: 24901457 Free PMC article. - In vivo astrocyte-to-neuron reprogramming for central nervous system regeneration: a narrative review.
Talifu Z, Liu JY, Pan YZ, Ke H, Zhang CJ, Xu X, Gao F, Yu Y, Du LJ, Li JJ. Talifu Z, et al. Neural Regen Res. 2023 Apr;18(4):750-755. doi: 10.4103/1673-5374.353482. Neural Regen Res. 2023. PMID: 36204831 Free PMC article. Review. - ASTROCYTES: EMERGING STARS IN LEUKODYSTROPHY PATHOGENESIS.
Lanciotti A, Brignone MS, Bertini E, Petrucci TC, Aloisi F, Ambrosini E. Lanciotti A, et al. Transl Neurosci. 2013 Jun 1;4(2):10.2478/s13380-013-0118-1. doi: 10.2478/s13380-013-0118-1. Transl Neurosci. 2013. PMID: 24340223 Free PMC article. - Advances in Transcription Factors Related to Neuroglial Cell Reprogramming.
Liu K, Ma W, Li C, Li J, Zhang X, Liu J, Liu W, Wu Z, Zang C, Liang Y, Guo J, Li L. Liu K, et al. Transl Neurosci. 2020 Feb 20;11:17-27. doi: 10.1515/tnsci-2020-0004. eCollection 2020. Transl Neurosci. 2020. PMID: 32161682 Free PMC article.
References
- Heinrich C., Gascón S., Masserdotti G., Lepier A., Sanchez R., Simon-Ebert T., Schroeder T., Götz M., Berninger B. Generation of subtype-specific neurons from postnatal astroglia of the mouse cerebral cortex. Nat. Protoc. 2011;6:214–228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous