Regional deficiencies in chaperone-mediated autophagy underlie α-synuclein aggregation and neurodegeneration - PubMed (original) (raw)
Regional deficiencies in chaperone-mediated autophagy underlie α-synuclein aggregation and neurodegeneration
Kristen A Malkus et al. Neurobiol Dis. 2012 Jun.
Abstract
In neurodegenerative diseases, it remains unclear why certain brain regions are selectively vulnerable to protein aggregation. In transgenic mice expressing human A53T α-synuclein, the brainstem and spinal cord develop the most prominent α-synuclein inclusions which correlate with age-dependent motor dysfunction. Herein we present the novel finding that this selective aggregation is in part dependent on the inability of chaperone-mediated autophagy (CMA) to effectively degrade α-synuclein in these brain regions. Lysosomal assays revealed that CMA activity was significantly decreased in aggregation-prone regions compared to the remainder of the brain. Previously, CMA activity has been shown to be proportional to levels of the CMA receptor Lamp-2a. Using antibodies, brain tissue from Lamp-2a null mice, enzymatic deglycosylation, and mass spectrometry, we identified Lamp2a as a novel 72kDa glycoprotein in the mouse brain. Examination of Lamp-2a levels revealed differences in expression across brain regions. The brainstem and the spinal cord had a more than three-fold greater levels of Lamp-2a as compared to regions less vulnerable to aggregation and exhibited a selective upregulation of Lamp-2a during development of α-synuclein inclusions. Despite this dynamic response of Lamp-2a, the levels of substrates bound to the brain lysosomes as well as the rates of substrate uptake and degradation were not proportional to the levels of Lamp-2a. These regional differences in CMA activity and Lamp-2a expression were found in both non-transgenic mice as well as A53T α-syn mice. Therefore, these are inherent variations and not a transgene-specific effect. However, differences in CMA activity may render select brain regions vulnerable to homeostatic dysfunction in the presence of stressors such as overexpression of human A53T α-syn. Collectively, the data provide a potential mechanism to explain the dichotomy of vulnerability or resistance that underlies brain regions during aggregate formation in neurodegenerative disease.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
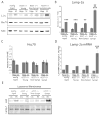
FIGURE 1
Reduced CMA activity in brain regions that are most vulnerable to α-syn inclusions. A, Purified lysosomes were extracted from the pathogenic regions of the brainstem and spinal cord (“Path”) or the remainder of the brain (“Non-Path”) of non-transgenic mice. The purified lysosomal fractions were verified to be free of mitochondria by SDS-PAGE/western blot with the mitochondrial marker cytochrome c oxidase I (MTCO1). B, Equal lysosomal loading and protease capacity across the two regions was verified by SDS-PAGE/western blot for Cathepsin D and quantified by densitometry. The resulting values were adjusted proportionally to the value of the non-path region to normalize intensity across experiments. C, 25 μg of purified lysosomes were incubated with 0.2 μg of purified human α-syn with Hsc70 and an energy regenerating system at 37°C. After the incubation, the samples were analyzed by SDS-PAGE/western blot with a human specific α-syn antibody and quantified by densitometry. A condition of lysosomes burst by hypotonic shock was included as a control for the activity of lysosomal proteases in the two samples. As a control for CMA dependent uptake, a “No ATP/Hsc70” condition was included. An additional control condition involved incubation of the lysosomes at 4 °C during the reaction to prevent uptake. All values were held relative to a final condition in which the 0.2 μg of α-syn was incubated under the same conditions only without any lysosomes present. The brainstem and spinal cord have a decreased ability to degrade α-syn by CMA, a difference that is not due to any deficiencies in the degradation ability of the lysosomal proteases and is dependent on ATP and lysosomal uptake (**p<0.01 path versus non-path regions, t-test, intact lysosomes (n=10), all other conditions, (n=3))
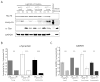
FIGURE 2
Lamp-2a characterization in the mouse brain. A, The brain and the spinal cord from non-transgenic littermates of the A53T α-syn mice were analyzed by SDS-PAGE/western blot for Lamp-2a (Invitrogen 51-2200). Two distinct bands were identified at 96kDa (asterisk) and 72kDa (arrow). B, The brainstem, spinal cord, and remainder of the brain excluding these regions “Brain (−)BS” were dissected from non-transgenic littermates of the A53T α-syn mice or from Lamp-2 knockout mice. This tissue was analyzed by SDS-PAGE/western blot for Lamp-2a using the Invitrogen #51-2200 Lamp-2a antibody. The 96kDa band recognized by the antibody was present in the Lamp-2 knockout mice (asterisk), while the 72kDa band is specific to Lamp-2a (arrow). C, The hippocampus (Hipp) and spinal cord (SC) of an non-transgenic mouse were incubated with a cocktail of N- and O- linked deglycosylating enzymes (PNGaseF; α-2(3,6,8,9) Neuraminidase; O-Glycosidase; β(1–4) Galactosidase; β-N-Acetylglucosaminidase; Endoglycosidase H). After incubation, the enzymes were heat inactivated, and the samples were analyzed by SDS-PAGE/western blot for Lamp-2a. α-Tubulin was used as a loading control. D, The brains of non-transgenic mice were dissected into eight specific regions and analyzed by SDS-PAGE/western blot for Lamp-2a. NSE served as a loading control. (SN - substantia nigra; OlfB - olfactory bulbs; Str - striatum; Hipp - hippocampus; Ctx - cortex; Crb - cerebellum; BS - brainstem; SC - spinal cord). E, The intensity of the immunoreactive band of Lamp-2a was quantified by densitometry relative to the intensity of NSE. The resulting values were adjusted proportionally to the value of the hippocampus to normalize intensity across experiments. Lamp-2a is expressed most prominently in the brainstem and the spinal cord (n=3) (***p<0.001, SC or BS vs SN, OlfB, Str, Hipp, Ctx, or Crb, one way ANOVA with Tukey’s post-hoc test).
FIGURE 3
Increased levels of Lamp-2a in the spinal cord during the onset of symptoms in transgenic A53T α-syn mice. A, The hippocampus (Hipp) and the spinal cord (SC) from symptomatic A53T α-syn transgenic mice (sympt), age matched non-symptomatic A53T α-syn transgenic mice (non-sympt), age matched non-transgenic mice (nonTg), and young A53T α-syn transgenic mice (2.5mo) (young) were analyzed by SDS-PAGE/western blot for the CMA components Lamp-2a and Hsc70. NSE was used as a loading control. B, The intensity of the immunoreactive band of Lamp-2a was quantified by densitometry relative to the intensity of NSE. The resulting values were adjusted proportionally to the value of the non-transgenic spinal cord to normalize intensity across experiments. This reinforced the increased levels of lamp-2a in the spinal cord relative to the hippocampus but revealed no effect of age or transgene expression on expression. Upon the onset of symptom development there was a selective increase in Lamp-2a in the spinal cord (n=3) (**p<0.01, ***p<0.001, Levels of Lamp-2a in SC versus Hipp within a particular condition of mice; ‡‡ p<0.001, Levels of Lamp-2a in the sympt SC versus non-Tg SC, young SC, and non-sympt SC, one-way ANOVA with Tukey’s post-hoc test). C, The intensity of the immunoreactive band of Hsc70 was quantified by densitometry relative to the intensity of NSE. The resulting values were adjusted proportionally to the value of the young hippocampus to normalize intensity across experiments. No significant differences in Hsc70 expression were found across conditions. D, Quantitative PCR with a primer against Lamp-2a and a primer against neuron specific enolase (NSE) as a control was performed on cDNA synthesized from RNA extracted from same conditions described above. Levels of Lamp-2a mRNA were held relative to values for the non-transgenic hippocampus. Comparison across the groups of mice revealed no change in mRNA levels in the hippocampus, but in the spinal cord there was a significant increase in Lamp-2a mRNA in the symptomatic A53T α-syn transgenic mice relative to the other conditions (n=3) (**p<0.01, sympt SC versus non-Tg SC, young SC, and non-sympt SC, *** p<0.001, sympt SC versus Hipp, one-way ANOVA with Tukey’s post-hoc test). E, Lamp-2a is localized in lysosomal membranes. Crude lysosomal extract (light M+L fraction) was obtained from the cortex or brainstem of symptomatic A53T α-syn transgenic mice, age matched non-symptomatic A53T α-syn transgenic mice, age matched non-transgenic mice, and 2.5 months of age (young) A53T α-syn transgenic mice. Lysosomes were burst by hypotonic shock and the membranes were separated from the lumen by ultracentrifugation. The lysosomal membranes were analyzed by SDS-PAGE/western blot for Lamp-2a and Lamp-1.
FIGURE 4
Association of substrates with lysosomes is not proportional to the levels of Lamp-2a at the lysosomal membrane. A, Crude lysosomal extracts (light M+L fraction) were obtained from the cortex (Ctx) and the brainstem (BS) of symptomatic A53T α-syn transgenic mice, age matched non-symptomatic A53T α-syn transgenic mice, age matched non-transgenic mice, and 2.5 months of age (young) A53T α-syn transgenic mice. The extracted lysosomes were analyzed by SDS-PAGE/western Blot alongside non-fractionated homogenate from the whole brains of non-transgenic mice with an antibodies against Hsc70, Lamp-2a, human α-syn, and GAPDH. B, Intensity of α-syn and Lamp-2a immunoreactive bands were quantified by densitometry. The intensity of α-syn was then held relative to the intensity of Lamp-2a from the same sample. No band for α-syn was present in the non-transgenic mice, showing that the band is specific for transgenic A53T human α-syn, and was excluded from the report of densitometric analysis. α-Syn showed decreased association with lysosomes relative to the levels of Lamp-2a in the brainstem compared to the cortex across all conditions (n=4) (**p<0.01, ***p<0.001, BS versus Ctx, one-way ANOVA with Tukey’s post-hoc test). C, Immunoreactive bands of lysosomal extracts were analyzed as in (C) for GAPDH. GAPDH showed decreased association with lysosomes relative to levels of Lamp-2a in the brainstem compared to the cortex across all conditions (n=4) (**p<0.01, ***p<0.001, BS versus Ctx, one-way ANOVA with Tukey’s post-hoc test).
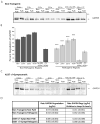
FIGURE 5
Rates of CMA substrate degradation across brain regions are not proportional to levels of Lamp-2a. A, Purified lysosomes were extracted from the pathogenic regions of the brainstem and spinal cord (“Path”) or the remainder of the brain (“Non-Path”) of non-transgenic mice. To determine the degradation of GAPDH endogenously associated with lysosomes, 75ug lysosomes were purified from non-transgenic mice. The lysosomes were then incubated for the indicated timepoints at 37°C with an energy regenerating system. After the incubation, the samples were analyzed by SDS-PAGE/western blot with a GAPDH antibody. As a control for CMA dependent uptake, a “No ERS” condition was included where the ATP energy regenerating system was not included. An additional control condition involved incubation of the lysosomes at 4°C during the reaction to prevent uptake. B, the immunoreactive bands in (A) were quantified by densitometry. All values were held relative to a condition in which the lysosomes from each region were immediately frozen (0 min). The pathogenic regions of non-transgenic mice have a decreased ability to degrade endogenous GAPDH by CMA, a difference that is dependent on ATP and lysosomal uptake (n=3, ***p<0.001, Non-Path vs Path for each timepoint, one-way ANOVA with Tukey’s post-hoc test). C, The endogenous lysosomal degradation of GAPDH was assessed for symptomatic A53T+/+ α-synuclein mice as in (A). The pathogenic regions of the A53T α-synuclein mice display comparable degradation of endogenous GAPDH compared to the non-pathogenic regions (n=3). D, The rate of GAPDH degradation was determined by comparing the intensity of the bands of the 0 hr and 15 hr time points in (C) and (D) with known standards of purified GAPDH. The resulting values were then divided by the relative amounts of Lamp-2a present in the different conditions, as determined by western blot (n=3).
Similar articles
- Chaperone-mediated autophagy: roles in disease and aging.
Cuervo AM, Wong E. Cuervo AM, et al. Cell Res. 2014 Jan;24(1):92-104. doi: 10.1038/cr.2013.153. Epub 2013 Nov 26. Cell Res. 2014. PMID: 24281265 Free PMC article. Review. - Age-dependent accumulation of oligomeric SNCA/α-synuclein from impaired degradation in mutant LRRK2 knockin mouse model of Parkinson disease: role for therapeutic activation of chaperone-mediated autophagy (CMA).
Ho PW, Leung CT, Liu H, Pang SY, Lam CS, Xian J, Li L, Kung MH, Ramsden DB, Ho SL. Ho PW, et al. Autophagy. 2020 Feb;16(2):347-370. doi: 10.1080/15548627.2019.1603545. Epub 2019 Apr 14. Autophagy. 2020. PMID: 30983487 Free PMC article. - Lysosome membrane lipid microdomains: novel regulators of chaperone-mediated autophagy.
Kaushik S, Massey AC, Cuervo AM. Kaushik S, et al. EMBO J. 2006 Sep 6;25(17):3921-33. doi: 10.1038/sj.emboj.7601283. Epub 2006 Aug 17. EMBO J. 2006. PMID: 16917501 Free PMC article. - Lysosomal-associated membrane protein 2 isoforms are differentially affected in early Parkinson's disease.
Murphy KE, Gysbers AM, Abbott SK, Spiro AS, Furuta A, Cooper A, Garner B, Kabuta T, Halliday GM. Murphy KE, et al. Mov Disord. 2015 Oct;30(12):1639-47. doi: 10.1002/mds.26141. Epub 2015 Jan 16. Mov Disord. 2015. PMID: 25594542 - Chaperone-mediated autophagy.
Dice JF. Dice JF. Autophagy. 2007 Jul-Aug;3(4):295-9. doi: 10.4161/auto.4144. Epub 2007 Jul 15. Autophagy. 2007. PMID: 17404494 Review.
Cited by
- Dysfunction of Cellular Proteostasis in Parkinson's Disease.
Lehtonen Š, Sonninen TM, Wojciechowski S, Goldsteins G, Koistinaho J. Lehtonen Š, et al. Front Neurosci. 2019 May 10;13:457. doi: 10.3389/fnins.2019.00457. eCollection 2019. Front Neurosci. 2019. PMID: 31133790 Free PMC article. Review. - Molecular chaperones and protein folding as therapeutic targets in Parkinson's disease and other synucleinopathies.
Ebrahimi-Fakhari D, Saidi LJ, Wahlster L. Ebrahimi-Fakhari D, et al. Acta Neuropathol Commun. 2013 Dec 5;1(1):79. doi: 10.1186/2051-5960-1-79. Acta Neuropathol Commun. 2013. PMID: 24314025 Free PMC article. Review. - Overexpression of alpha-synuclein at non-toxic levels increases dopaminergic cell death induced by copper exposure via modulation of protein degradation pathways.
Anandhan A, Rodriguez-Rocha H, Bohovych I, Griggs AM, Zavala-Flores L, Reyes-Reyes EM, Seravalli J, Stanciu LA, Lee J, Rochet JC, Khalimonchuk O, Franco R. Anandhan A, et al. Neurobiol Dis. 2015 Sep;81:76-92. doi: 10.1016/j.nbd.2014.11.018. Epub 2014 Dec 8. Neurobiol Dis. 2015. PMID: 25497688 Free PMC article. - The role of chaperone-mediated autophagy in neurotoxicity induced by alpha-synuclein after methamphetamine exposure.
Sun L, Lian Y, Ding J, Meng Y, Li C, Chen L, Qiu P. Sun L, et al. Brain Behav. 2019 Aug;9(8):e01352. doi: 10.1002/brb3.1352. Epub 2019 Jul 9. Brain Behav. 2019. PMID: 31286692 Free PMC article. - Chaperone-mediated autophagy: roles in disease and aging.
Cuervo AM, Wong E. Cuervo AM, et al. Cell Res. 2014 Jan;24(1):92-104. doi: 10.1038/cr.2013.153. Epub 2013 Nov 26. Cell Res. 2014. PMID: 24281265 Free PMC article. Review.
References
- Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, Caballero C, Ferrer I, Obeso JA, Schapira AH. Chaperone-mediated autophagy markers in Parkinson disease brains. Arch Neurol. 2010;67(12):1464–72. - PubMed
- Andrejewski N, Punnonen EL, Guhde G, Tanaka Y, Lüllmann-Rauch R, Hartmann D, von Figura K, Saftig P. Normal lysosomal morphology and function in LAMP-1-deficient mice. J Biol Chem. 1999;274(18):12692–701. - PubMed
- Braak H, Bohl JR, Müller CM, Rüb U, de Vos RA, Del Tredici K. Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord. 2006;21(12):2042–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous