Human primary lung endothelial cells in culture - PubMed (original) (raw)
Human primary lung endothelial cells in culture
Suzy A A Comhair et al. Am J Respir Cell Mol Biol. 2012 Jun.
Abstract
Pulmonary endothelial functions are critical to maintain the low pressure of the pulmonary circulation and effective diffusion capacity of the lung. To investigate pulmonary endothelial cell biology in healthy or diseased lungs, we developed methods to harvest and culture pure populations of primary pulmonary arterial endothelial cells and microvascular endothelial cells from human lung explanted at time of transplantation or from donor lungs not used in transplantation. The purity and characteristics of cultured endothelial cells is ascertained by morphologic criteria using phase contrast and electron microscopy; phenotypic expression profile for endothelial specific proteins such as endothelial nitric oxide synthase, platelet/endothelial cell adhesion molecule, and von Willbrand factor; and endothelial function assays such as Dil-acetylated low-density lipoprotein uptake and tube formation. This detailed method provides researchers with the ability to establish cells for molecular, genetic, and biochemical investigation of human pulmonary vascular diseases.
Figures
Figure 1.
Morphology of endothelial cells and smooth muscle cells. Phase-contrast microscopic analysis of primary pulmonary arterial endothelial cells shows a rosette cobblestone morphology (arrow) (A). Confluent pulmonary arterial endothelial cells (B) and microvascular endothelial cells (C) at passage 5 demonstrating the characteristic cobblestone appearance of endothelial cells. Phase-contrast microscopic of primary pulmonary arterial smooth muscle cells (D). Endothelial cells and smooth muscle cells shown in this figure were initiated from pulmonary arterial hypertension (PAH) lungs. There were no morphological differences between endothelial cells or smooth muscle cells derived from PAH or control lungs.
Figure 2.
Ultrastructure of control and PAH pulmonary arterial endothelial cells and PAH lung tissue. Electromicroscopy of culture control (A, D) and PAH pulmonary arterial endothelial cells (B, E) and PAH lung tissue (C, F). Ultrastructure detail of endothelial cells in vitro (A, B, D, E) and in vivo (C, F) (scale bar: 1 μm). Weibel-Palade bodies in cultured endothelial cell (E) and endothelial cell of lung tissue (F) (arrows) (scale bar: 250 nm). M = mitochondria; N = nucleus; RBC = red blood cells.
Figure 3.
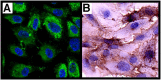
Phenotype of primary pulmonary arterial endothelial cells. Characterization of pulmonary arterial cells ascertained by morphologic and immunohistochemistry analyses of endothelial markers (CD31, von Willebrand factor). (A) Immunofluorescence for von Willebrand factor (green) and (B) CD31 (brown) by immunohistochemistry. Representative image is from PAH cells. Hence, control pulmonary arterial endothelial cells have the same phenotype.
Figure 4.
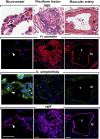
Endothelial cell types in vessels and plexiform lesions in PAH. Sequential sections for hematoxylin and eosin (H&E), Helix pomatia (red), Griffonia simplicifolia (green), and vWF (purple). H. pomatia binds to endothelium of muscular artery, whereas G. simplicifolia binds to endothelium of microvessels. Red blood cells also bind G. simplicifolia. White arrows and black arrows indicate capillaries. M = smooth muscle; V = vascular lumen of large muscular artery. Scale bar: 100 μm.
Figure 5.
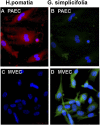
Lectin binding of microvascular endothelial cells (MVECs) and pulmonary artery endothelial cells (PAECs) in vitro. (A_–_D) Immunofluorescent H. pomatia (red) binds PAECs in vitro (A) but not MVECs (C). G. simplicifolia (green) binds MVECs in culture (D) but not to PAECs (B). Representative image is from PAH cells. Hence, control PAECs have the same phenotype.
Figure 6.
Endothelial nitric oxide synthase (eNOS) expression in PAECs and MVECs. (A, B) Immunohistochemistry shows strong eNOS staining in the muscular vessels where the staining in alveolar capillaries (arrows) is absent. The plexiform lesion (A) is lined with endothelial cells that are mostly positive for eNOS, but some cells are negative. (C, D) Sequential section confirms endothelial cells by CD31 and in plexiform lesions. (E) Western blot analysis shows that control and PAH PAECs express eNOS, whereas MVECs have undetectable or weak expression of eNOS. C = capillaries; M = smooth muscle; V = vessel.
Figure 7.
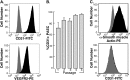
Flow cytometric analysis of endothelial cell surface antigen expression on PAECs. Confluent cell cultures were harvested by trypsinization and stained for cell surface expression of endothelial cell specific markers CD31 and vascular endothelial growth factor receptor 2. (A) Representative profiles for each staining is shown. Gray histograms indicate staining with isotype-matched control antibodies. (B) Purity of primary PAECs, as measured by %CD31-positive cells, increases during passaging of the cells. Cells were more than 95% CD31 positive by passage 4. Passage 1, n = 7; passage 2, n = 14; passage 3, n = 15; passage 4, n = 13; passage 5, n = 31. (C) The smooth muscle cells cultured from pulmonary arteries stained positive for smooth muscle cell α-actin and negative for CD31. Representative profiles for each staining is shown. Gray histograms indicate staining with isotype-matched control antibodies. Representative image is from PAH cells. Control pulmonary arterial endothelial cells have the same phenotype.
Figure 8.
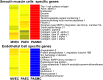
Gene expression analysis. Hierarchical clustering of gene expression array data from PAECs (n = 14), MVECs (n = 5), and pulmonary artery smooth muscle cells (PASMCs) (n = 4) demonstrates that PAECs and MVECs have closely related gene expression signatures but are distinctly different from PASMCs. Color coding denotes relative gene expression on a continuous scale from blue (lowest expression), through yellow (similar expression levels) to red (highest).
Figure 9.
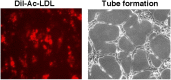
Functional assays. (A) Accumulation of acetylated low-density lipoprotein (Dil-Ac-LDL) by primary PAECs (passage 5). (B) Capillary-like tubule formation produced by primary PAECs 8 hours after plating onto Matrigel. Representative image is from control cells. Hence, PAH PAECs have the same phenotype.
Similar articles
- Activation-dependent isolation and culture of murine pulmonary microvascular endothelium.
Gerritsen ME, Shen CP, McHugh MC, Atkinson WJ, Kiely JM, Milstone DS, Luscinskas FW, Gimbrone MA Jr. Gerritsen ME, et al. Microcirculation. 1995 Aug;2(2):151-63. doi: 10.3109/10739689509146763. Microcirculation. 1995. PMID: 7497167 - Endothelial cell processing and alternatively spliced transcripts of factor VIII: potential implications for coagulation cascades and pulmonary hypertension.
Shovlin CL, Angus G, Manning RA, Okoli GN, Govani FS, Elderfield K, Birdsey GM, Mollet IG, Laffan MA, Mauri FA. Shovlin CL, et al. PLoS One. 2010 Feb 11;5(2):e9154. doi: 10.1371/journal.pone.0009154. PLoS One. 2010. PMID: 20174619 Free PMC article. - Isolation and maintenance of human pulmonary artery endothelial cells in culture isolated from transplant donors.
Visner GA, Staples ED, Chesrown SE, Block ER, Zander DS, Nick HS. Visner GA, et al. Am J Physiol. 1994 Oct;267(4 Pt 1):L406-13. doi: 10.1152/ajplung.1994.267.4.L406. Am J Physiol. 1994. PMID: 7943344 - Isolation and characterisation of human pulmonary microvascular endothelial cells from patients with severe emphysema.
Mackay LS, Dodd S, Dougall IG, Tomlinson W, Lordan J, Fisher AJ, Corris PA. Mackay LS, et al. Respir Res. 2013 Feb 20;14(1):23. doi: 10.1186/1465-9921-14-23. Respir Res. 2013. PMID: 23425195 Free PMC article. - Pulmonary endothelium: a dynamic interface.
Ryan US. Ryan US. Clin Invest Med. 1986;9(2):124-32. Clin Invest Med. 1986. PMID: 3015468 Review.
Cited by
- Altered expression and signal transduction of endothelin-1 receptors in heritable and idiopathic pulmonary arterial hypertension.
Yu J, Taylor L, Wilson J, Comhair S, Erzurum S, Polgar P. Yu J, et al. J Cell Physiol. 2013 Feb;228(2):322-9. doi: 10.1002/jcp.24132. J Cell Physiol. 2013. PMID: 22688668 Free PMC article. - Vascular Endothelial Growth Factor Receptor 3 Regulates Endothelial Function Through β-Arrestin 1.
Ma Z, Yu YR, Badea CT, Kovacs JJ, Xiong X, Comhair S, Piantadosi CA, Rajagopal S. Ma Z, et al. Circulation. 2019 Mar 26;139(13):1629-1642. doi: 10.1161/CIRCULATIONAHA.118.034961. Circulation. 2019. PMID: 30586762 Free PMC article. - ACTRIIA-Fc rebalances activin/GDF versus BMP signaling in pulmonary hypertension.
Yung LM, Yang P, Joshi S, Augur ZM, Kim SSJ, Bocobo GA, Dinter T, Troncone L, Chen PS, McNeil ME, Southwood M, Poli de Frias S, Knopf J, Rosas IO, Sako D, Pearsall RS, Quisel JD, Li G, Kumar R, Yu PB. Yung LM, et al. Sci Transl Med. 2020 May 13;12(543):eaaz5660. doi: 10.1126/scitranslmed.aaz5660. Sci Transl Med. 2020. PMID: 32404506 Free PMC article. - Dysregulation of the Long Noncoding RNA X-Inactive-Specific Transcript Expression in Male Patients with Pulmonary Arterial Hypertension.
Carman BL, Qin S, Predescu DN, Jana M, Cortese R, Aldred MA, Gozal D, Mokhlesi B, Predescu SA. Carman BL, et al. Am J Pathol. 2024 Aug;194(8):1592-1606. doi: 10.1016/j.ajpath.2024.04.005. Epub 2024 May 3. Am J Pathol. 2024. PMID: 38705381 - Comprehensive phenotyping of endothelial cells using flow cytometry 2: Human.
Grant D, Wanner N, Frimel M, Erzurum S, Asosingh K. Grant D, et al. Cytometry A. 2021 Mar;99(3):257-264. doi: 10.1002/cyto.a.24293. Epub 2020 Dec 23. Cytometry A. 2021. PMID: 33369145 Free PMC article. Review.
References
- Archer S, Rich S. Primary pulmonary hypertension: a vascular biology and translational research “work in progress”. Circulation 2000;102:2781–2791 - PubMed
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980;288:373–376 - PubMed
- Ignarro LJ. Nitric oxide-mediated vasorelaxation. Thromb Haemost 1993;70:148–151 - PubMed
- King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res 2004;67:139–151 - PubMed
MeSH terms
Grants and funding
- R37 HL060917/HL/NHLBI NIH HHS/United States
- R01 HL060917/HL/NHLBI NIH HHS/United States
- P01 HL103453/HL/NHLBI NIH HHS/United States
- R01 HL115008/HL/NHLBI NIH HHS/United States
- U10 HL109250/HL/NHLBI NIH HHS/United States
- R01 HL098199/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous