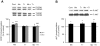In vivo manganese exposure modulates Erk, Akt and Darpp-32 in the striatum of developing rats, and impairs their motor function - PubMed (original) (raw)
doi: 10.1371/journal.pone.0033057. Epub 2012 Mar 13.
Aderbal S Aguiar Jr, Tanara V Peres, Mark W Lopes, Filipe M Gonçalves, Aline P Remor, Samantha C Lopes, Célso Pilati, Alexandra S Latini, Rui D S Prediger, Keith M Erikson, Michael Aschner, Rodrigo B Leal
Affiliations
- PMID: 22427945
- PMCID: PMC3302787
- DOI: 10.1371/journal.pone.0033057
In vivo manganese exposure modulates Erk, Akt and Darpp-32 in the striatum of developing rats, and impairs their motor function
Fabiano M Cordova et al. PLoS One. 2012.
Abstract
Manganese (Mn) is an essential metal for development and metabolism. However, exposures to high Mn levels may be toxic, especially to the central nervous system (CNS). Neurotoxicity is commonly due to occupational or environmental exposures leading to Mn accumulation in the basal ganglia and a Parkinsonian-like disorder. Younger individuals are more susceptible to Mn toxicity. Moreover, early exposure may represent a risk factor for the development of neurodegenerative diseases later in life. The present study was undertaken to investigate the developmental neurotoxicity in an in vivo model of immature rats exposed to Mn (5, 10 and 20 mg/kg; i.p.) from postnatal day 8 (PN8) to PN12. Neurochemical analysis was carried out on PN14. We focused on striatal alterations in intracellular signaling pathways, oxidative stress and cell death. Moreover, motor alterations as a result of early Mn exposure (PN8-12) were evaluated later in life at 3-, 4- and 5-weeks-of-age. Mn altered in a dose-dependent manner the activity of key cell signaling elements. Specifically, Mn increased the phosphorylation of DARPP-32-Thr-34, ERK1/2 and AKT. Additionally, Mn increased reactive oxygen species (ROS) production and caspase activity, and altered mitochondrial respiratory chain complexes I and II activities. Mn (10 and 20 mg/kg) also impaired motor coordination in the 3(rd), 4(th) and 5(th) week of life. Trolox™, an antioxidant, reversed several of the Mn altered parameters, including the increased ROS production and ERK1/2 phosphorylation. However, Trolox™ failed to reverse the Mn (20 mg/kg)-induced increase in AKT phosphorylation and motor deficits. Additionally, Mn (20 mg/kg) decreased the distance, speed and grooming frequency in an open field test; Trolox™ blocked only the decrease of grooming frequency. Taken together, these results establish that short-term exposure to Mn during a specific developmental window (PN8-12) induces metabolic and neurochemical alterations in the striatum that may modulate later-life behavioral changes. Furthermore, some of the molecular and behavioral events, which are perturbed by early Mn exposure are not directly related to the production of oxidative stress.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. Effects of short-term Mn exposure on metal accumulation in the hippocampus, striatum and cerebral cortex of immature rats.
The panels show the accumulation of Mn (A) and Fe (B). Rat pups were treated for five days (PN8-12) with saline (control; NaCl 0.9%) or MnCl2 at doses of 5, 10 or 20 mg/kg. The tissues were analyzed on PN14. Results represent mean ± S.E.M and are expressed in µg metal/g tissue derived from four independent experiments. Statistical analysis was performed by ANOVA followed by Duncan's test. * p<0.05, ** p<0.01, *** p<0.001 compared to control.
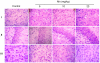
Figure 2. Histological evaluation of the brain of immature rats exposed to Mn in vivo.
The panel shows representative sections from eight independent experiments of (I) striatum, (II) hippocampus and (III) cerebral cortex from rats treated for five days (PN8-12) with saline (control; NaCl 0.9%) or MnCl2 at doses of 5, 10 or 20 mg/kg. The structures analyzed on PN14. The sections were stained with hematoxylin and eosin staining. Magnification ×40.
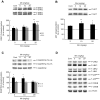
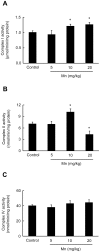
Figure 3. Effects of in vivo exposure to Mn for five days on the phosphorylation of MAPKs, AKT, CREB and DARPP-32 in the striatum of immature rats.
The panels show representative immunoblotting and quantification of phosphorylation of ERK1/2 (A), AKT (B), DARPP-32-Thr-34 and -Thr-75 (C) and JNK1/2, p38MAPK, CREB and β-Actin (D) from rats treated for five days (PN8-12) with saline (control; NaCl 0.9%) or MnCl2 at doses of 5, 10 or 20 mg/kg/day. The structures analyzed on PN14. Total and phosphorylated forms of each protein were detected by specific antibodies and the reaction was developed by chemiluminescence. The phosphorylation level of each protein was determined as a ratio of the O.D. of the phosphorylated band over the O.D. of the total band and the data are expressed as percentage of the control (considered as 100%) and the values are presented as mean ± S.E.M derived from twelve independent experiments. Statistical analysis was performed by ANOVA followed by Duncan's test. * p<0.05, ** p<0.01, *** p<0.001 compared to control.
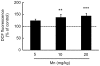
Figure 4. Mn induces oxidative stress in the striatum.
Oxidative stress was analyzed by DCF fluorescence in the striatum of young rats treated with Mn. The graphic shows the DCF fluorescence from rats treated for five days (PN8-12) with saline (control; NaCl 0.9%) or MnCl2 at doses of 5, 10 or 20 mg/kg/day. The structures were analyzed on PN14. The data are expressed as percentage of the control and the values are mean ± S.E.M derived from eight independent experiments. Statistical analysis was performed by ANOVA followed by Duncan's test. ** p<0.01, *** p<0.001 compared to control.
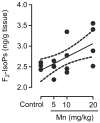
Figure 5. Mn induces F2-IsoPs production.
Striatum from immature rats (PN14) exposed in vivo to Mn (PN8-12) were evaluated for F2-IsoPs levels expressed as ng/g tissue. The graphic shows a dose-dependent increase of F2-IsoPs levels by MnCl2 (5, 10 or 20 mg/kg/day). The values were obtained from four independent experiments. Pearson's correlation showed r2 = 0.38 with p = 0.011.
Figure 6. Effects of Mn on striatal activity of mitochondrial respiratory chain complexes in immature rats.
(A) Activity of mitochondrial complex I. (B) Activity of mitochondrial complex II. (C) Activity of mitochondrial complex IV. Rat pups were treated for five days (PN8-12) with saline (control; NaCl 0.9%) or MnCl2 at doses of 5, 10 or 20 mg/kg. Activities were analyzed on PN14. Results represent mean ± S.E.M and are expressed as nmol min−1/mg protein−1 or mmol min−1/mg protein−1 derived from four independent experiments. Statistical analysis was performed by ANOVA followed by Duncan's test. * p<0.05 compared to control.
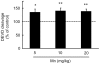
Figure 7. Mn treatment stimulates caspase activity in the striatum of immature rats.
Caspase activities were measured by DEVD cleavage. The panel shows the DEVD cleavage test from rats treated for five days (PN8-12) with saline (control; NaCl 0.9%) or MnCl2 at doses of 5, 10 or 20 mg/kg/day. Activities were analyzed on PN14. Results represent mean ± S.E.M and are expressed as percentage of control (100%) derived from eight independent experiments. Statistical analysis was performed by ANOVA followed by Duncan's test. * p<0.05, ** p<0.01 compared to control.
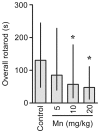
Figure 8. Mn exposure on PN8-12 causes later life onset motor deficits in rats.
To evaluate motor coordination, control animals (saline) and rats treated with Mn (PN8-12) were tested on 22, 29 and 36 days of age (3, 4 and 5 week-old) on the rotarod task. The graphic shows the overall latency for falling in rats treated with saline (control; NaCl 0.9%) or MnCl2 at doses of 5, 10 or 20 mg/kg/day. Results represent median ± interquartile range and are expressed as seconds (s) to latency for falling derived from twelve independent experiments. Statistical analysis was performed by Kruskal-Wallis followed by Dunn's post-hoc test. * p<0.05 compared to control.
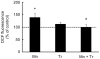
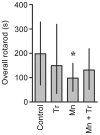
Figure 9. Trolox™ blocked the Mn-induced striatal oxidative stress.
DCF fluorescence in the striatum from rats treated on PN8-12 with saline (control; NaCl 0.9%), MnCl2 20 mg/kg (Mn), Trolox™ 1 mg/kg (Tr) or MnCl2 20 mg/kg plus Trolox™ 1 mg/kg (Mn + Tr) was analyzed on PN14. Results represent mean ± S.E.M and are expressed as percent of control (100%) to DCF fluorescence derived from eight independent experiments. Statistical analysis was performed by ANOVA followed by Duncan's test. * p<0.05, compared to control; # p<0.05 compared to MnCl2 20 mg/kg group.
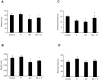
Figure 10. Effects of Trolox™ on the phosphorylation of ERK1/2 and AKT in the striatum of immature rats exposed to Mn.
The panels (A) and (B) show representative immunoblotting and quantification of ERK1/2 and AKT phosphorylation, respectively, from rats treated for five days (PN8-12) with saline (control; NaCl 0.9%), MnCl2 20 mg/kg (Mn), Trolox™ 1 mg/kg (Tr) or MnCl2 20 mg/kg plus Trolox™ 1 mg/kg (Mn + Tr). The tissues were harvested from the rats on PN14. Total and phosphorylated forms of each protein were detected by specific antibodies and the reaction was developed by chemiluminescence. The phosphorylation of each protein was determined as a ratio of the O.D. of the phosphorylated band over the O.D. of the total band and the data are expressed as percentage of the control (considered as 100%) and the values are presented as mean ± S.E.M derived from twelve independent experiments. Statistical analysis was performed by ANOVA followed by Duncan's test. * p<0.05, ** p<0.01, *** p<0.001 compared to control; # p<0.05 and ### p<0.001 compared to MnCl2 20 mg/kg group.
Figure 11. Effects of Trolox™ on the motor coordination in immature rats exposed to Mn.
Rats were treated for five days (PN8-12) with saline (control; NaCl 0.9%), MnCl2 20 mg/kg (Mn), Trolox™ 1 mg/kg (Tr) or MnCl2 20 mg/kg plus Trolox™ 1 mg/kg (Mn + Tr), and tested on 22, 29 and 36 days of age (3, 4 and 5 week old) at the rotarod task. Results represent median ± interquartile range and are expressed as seconds (s) to latency for falling derived from twelve independent experiments. Statistical analysis was performed by Kruskal-Wallis followed by Dunn's post-hoc test. * p<0.05 compared to control.
Figure 12. Motor behavioral effects in rats exposed to manganese and Trolox™.
The animals were tested in the circular open field for 10 min to evaluate possible motor changes induced by different treatments used. The panels show the parameters analyzed from rats treated for five days (PN8-12) with saline (control; NaCl 0.9%), MnCl2 20 mg/kg (Mn), Trolox™ 1 mg/kg (Tr) or MnCl2 20 mg/kg plus Trolox™ 1 mg/kg (Mn + Tr). Panel (A) show the distance (m), (B) speed (m/s), (C) grooming frequency and (D) rearing frequency. Results represent mean ± S.E.M derived from twelve independent experiments. Statistical analysis was performed by ANOVA followed by Newman-Keuls test. * p<0.05 compared to control; # p<0.05 compared to MnCl2 20 mg/kg group.
Similar articles
- Manganese-exposed developing rats display motor deficits and striatal oxidative stress that are reversed by Trolox.
Cordova FM, Aguiar AS Jr, Peres TV, Lopes MW, Gonçalves FM, Pedro DZ, Lopes SC, Pilati C, Prediger RD, Farina M, Erikson KM, Aschner M, Leal RB. Cordova FM, et al. Arch Toxicol. 2013 Jul;87(7):1231-44. doi: 10.1007/s00204-013-1017-5. Epub 2013 Feb 6. Arch Toxicol. 2013. PMID: 23385959 Free PMC article. - DARPP-32 and Akt regulation in ethanol-preferring AA and ethanol-avoiding ANA rats.
Nuutinen S, Kiianmaa K, Panula P. Nuutinen S, et al. Neurosci Lett. 2011 Sep 26;503(1):31-6. doi: 10.1016/j.neulet.2011.08.002. Epub 2011 Aug 7. Neurosci Lett. 2011. PMID: 21843598 - Sodium P-Aminosalicylic Acid Improved Manganese-Induced Learning and Memory Dysfunction via Restoring the Ultrastructural Alterations and γ-Aminobutyric Acid Metabolism Imbalance in the Basal Ganglia.
Ou CY, Luo YN, He SN, Deng XF, Luo HL, Yuan ZX, Meng HY, Mo YH, Li SJ, Jiang YM. Ou CY, et al. Biol Trace Elem Res. 2017 Mar;176(1):143-153. doi: 10.1007/s12011-016-0802-4. Epub 2016 Aug 5. Biol Trace Elem Res. 2017. PMID: 27491492 - DARPP-32 40 years later.
Girault JA, Nairn AC. Girault JA, et al. Adv Pharmacol. 2021;90:67-87. doi: 10.1016/bs.apha.2020.09.004. Epub 2020 Dec 2. Adv Pharmacol. 2021. PMID: 33706939 Review. - Role for calcium signaling in manganese neurotoxicity.
Ijomone OM, Aluko OM, Okoh COA, Martins AC Jr, Aschner M. Ijomone OM, et al. J Trace Elem Med Biol. 2019 Dec;56:146-155. doi: 10.1016/j.jtemb.2019.08.006. Epub 2019 Aug 22. J Trace Elem Med Biol. 2019. PMID: 31470248 Review.
Cited by
- Neurodegenerative Diseases: Implications of Environmental and Climatic Influences on Neurotransmitters and Neuronal Hormones Activities.
Ayeni EA, Aldossary AM, Ayejoto DA, Gbadegesin LA, Alshehri AA, Alfassam HA, Afewerky HK, Almughem FA, Bello SM, Tawfik EA. Ayeni EA, et al. Int J Environ Res Public Health. 2022 Sep 30;19(19):12495. doi: 10.3390/ijerph191912495. Int J Environ Res Public Health. 2022. PMID: 36231792 Free PMC article. Review. - Manganese Is Essential for Neuronal Health.
Horning KJ, Caito SW, Tipps KG, Bowman AB, Aschner M. Horning KJ, et al. Annu Rev Nutr. 2015;35:71-108. doi: 10.1146/annurev-nutr-071714-034419. Epub 2015 May 13. Annu Rev Nutr. 2015. PMID: 25974698 Free PMC article. Review. - Prenatal manganese and cord blood mitochondrial DNA copy number: Effect modification by maternal anemic status.
Kupsco A, Sanchez-Guerra M, Amarasiriwardena C, Brennan KJM, Estrada-Gutierrez G, Svensson K, Schnaas L, Pantic I, Téllez-Rojo MM, Baccarelli AA, Wright RO. Kupsco A, et al. Environ Int. 2019 May;126:484-493. doi: 10.1016/j.envint.2019.02.029. Epub 2019 Mar 5. Environ Int. 2019. PMID: 30849576 Free PMC article. - Role of early life exposure and environment on neurodegeneration: implications on brain disorders.
Modgil S, Lahiri DK, Sharma VL, Anand A. Modgil S, et al. Transl Neurodegener. 2014 Apr 29;3:9. doi: 10.1186/2047-9158-3-9. eCollection 2014. Transl Neurodegener. 2014. PMID: 24847438 Free PMC article. Review. - Oxidative Stress as a Common Key Event in Developmental Neurotoxicity.
Nishimura Y, Kanda Y, Sone H, Aoyama H. Nishimura Y, et al. Oxid Med Cell Longev. 2021 Jul 19;2021:6685204. doi: 10.1155/2021/6685204. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34336113 Free PMC article. Review.
References
- Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann N Y Acad Sci. 2004;1012:115–128. - PubMed
- Perl DP, Olanow CW. The neuropathology of manganese-induced Parkinsonism. J Neuropathol Exp Neurol. 2007;66:675–682. - PubMed
- Roth JA. Are there common biochemical and molecular mechanisms controlling manganism and parkisonism. Neuromolecular Med. 2009;11:281–296. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous