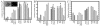Alterations in mast cell frequency and relationship to angiogenesis in the rat mammary gland during windows of physiologic tissue remodeling - PubMed (original) (raw)
Alterations in mast cell frequency and relationship to angiogenesis in the rat mammary gland during windows of physiologic tissue remodeling
Robert A Ramirez et al. Dev Dyn. 2012 May.
Abstract
Background: The mammary epithelium undergoes proliferation and regression accompanied by remodeling of the fibrocellular and vascular stroma. Mast cells are abundant in these compartments and have been implicated in remodeling during wound healing and cancer progression. The purpose of this study was to test the hypothesis that mast cell abundance correlates with physiologic mammary tissue remodeling during estrous cycling, lactogenesis (pregnancy and lactation) and involution.
Results: Mast cell and capillary frequency were quantified in the stroma surrounding ducts and lobules from mammary glands of rats. During estrous cycling, periductal mast cell numbers were unchanged, but lobule-associated mast cells significantly increased in the regressive phase of diestrus II. During lactogenesis, lobular stroma mast cells peaked early in pregnancy, at D2, followed by a significant decrease throughout lactation. Involution was associated with a rapid return in mast cell numbers, similar to diestrus II. Lobular vascularization peaked during the state of metestrus, when limited secretory differentiation occurs. Lobular angiogenesis peaked at D7 of pregnancy, regressed, and then returned to high levels during lactation and early involution, when secretory differentiation is high.
Conclusions: These results suggest mast cells are predominantly associated with regressive lobular remodeling during cycling and involution, whereas angiogenesis is predominantly associated with secretory differentiation.
Copyright © 2012 Wiley Periodicals, Inc.
Figures
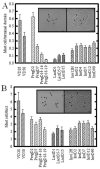
Fig. 1
Mast cell frequency during mammary gland development and lactogenic differentiation. A,B: Terminal ductal lobular units (A) and ducts (B) were analyzed for mast cell number. Panel (A), inset, shows toluidine blue stained mast cells (arrowheads) associated with lobular stroma; the right panel shows the same field under phase contrast. A: Alveolar-associated mast cells were highest in prepubertal (VD28), and slightly but not significantly decreased in peripubertal virgin mammary gland (VD50). Mast cells were initially increased at day 2 of pregnancy, and thereafter significantly and progressively decreased throughout pregnancy. A trend toward increasing mast cell numbers was seen during lactation, with a further progressive increase during early involution. By day 2 of involution, mast cells had returned to resting mammary gland (VD50) levels. Panel (B), inset, shows toluidine blue stained mast cells (arrowheads) surrounding a duct; the right panel shows the same field under phase contrast. B: Mast cell number in ductal stroma was highest in prepubertal (VD28 [virgin day 28]) mammary gland. Thereafter, numbers were decreased and no significant trends were seen during pregnancy (PD18–19), lactation, or involution. Quantitative data shows means ± SEM of total number of fields. P < 0.05 was determined to be significant. Scale bar = 10 μm.
Fig. 2
Mast cell abundance in toluidine-blue sections throughout postnatal mammary gland development. A–L: Figure 2 demonstrates the histologic appearance of the darkly stained mast cells (arrows) associated with lobular ductules, whether in resting nulliparous (A,B) or parous glands (C) or during pregnancy (D–F), lactation (G–I), or involution (J–L).
Fig. 3
Capillary frequency in lobules during mammary gland development and lactogenic differentiation. A: Inset shows appearance of hematoxylin and eosin (H&E) -stained paraffin sections of mammary gland under bright field (left panel) and epifluorescent (right panel) illumination. Lobular ductules (arrows) and the number of red blood cell-containing capillaries (arrowhead) were assessed for each lobule, and the number of lobular capillaries was normalized per lobular ductule. Magnification bar represents 20 mm. Asterisks indicate time points where the entire lobule was too large to be imaged in one field, and so data are normalized per field. B: Bars represent average number of ductules either per lobule, or per field at time points when the lobular size exceeded a microscopic field of view (bars with asterisk). C: Capillary number normalized per lobular ductule to remove effects of total lobule size. Capillary number was highest throughout lactation and until D2 involution, significantly decreased at days 4 and 12, and thereafter returned to resting levels at day 90. Statistical analysis was performed using All Pairwise Multiple Comparison Procedures (Dunn’s Method), which sets significance as P < 0.05. Virgin, pregnancy, and parous (d90 involution) time points were not significantly different. Normalized lobular vascularization at day 1 of lactation was significantly greater than both virgin time points, pregnancy time points except day 7, and greater than day 90 involution glands. Day 5–7 lactation was significantly greater than all virgin and pregnancy time points, as well as day 90 involution. Involution at 12 hr, day 2, day 4, and day 12 postweaning were significantly different from all other time points except lactation. Day 12 involution was significantly different from virgin day 50, pregnancy day 2, and day 18–19, and involution day 90; the lobular vascularization of the mammary gland at day 90 postinvolution (i.e., glands from parous rats) was not significantly different from the mammary glands of nulliparous virgin rats. Bars indicate the mean±SEM.
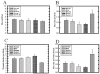
Fig. 4
A–D: Effect of estrous stage on mast cells associated with (A) ductal stroma, (B) terminal ductule lobular unit, (C) number of terminal ductules per lobule, and (D) mast cells per lobular ductule. A: No significant difference in mast cell number was seen associated with ductal stroma. B: In contrast, mast cells associated with lobules were significantly increased during diestrus II, compared with metestrus and diestrus I (P < 0.05). C: The number of terminal ductules per lobule was assessed, and dropped significantly during diestrus II, compared with all other estrous stages (P < 0.05). D: The number of mast cells normalized per lobular ductule for each field demonstrated that mast cells were significantly greatest in number during diestrus II, compared with all other estrous stages (P < 0.05). Bars indicate mean ± SEM.
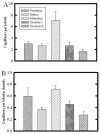
Fig. 5
Quantitative analysis of capillary number associated with lobules in the rat mammary gland throughout the estrous cycle. A: Number of capillaries per entire lobule was greatly increased during metestrus, compared with all other time points (P < 0.05). The capillary number during diestrus II was also significantly less than that during proestrus, estrus, and metestrus (P < 0.05). B: When capillary number was normalized per lobular ductule, capillaries per lobular ductule were significantly increased during metestrus, compared with all other stages; in addition, capillary number during diestrus II was significantly decreased compared with all other time points (P < 0.05). Bars indicate mean ± SEM.
Similar articles
- Postpartum breast involution reveals regression of secretory lobules mediated by tissue-remodeling.
Jindal S, Gao D, Bell P, Albrektsen G, Edgerton SM, Ambrosone CB, Thor AD, Borges VF, Schedin P. Jindal S, et al. Breast Cancer Res. 2014 Mar 28;16(2):R31. doi: 10.1186/bcr3633. Breast Cancer Res. 2014. PMID: 24678808 Free PMC article. - Conditional deletion of Stat3 in mammary epithelium impairs the acute phase response and modulates immune cell numbers during post-lactational regression.
Hughes K, Wickenden JA, Allen JE, Watson CJ. Hughes K, et al. J Pathol. 2012 May;227(1):106-17. doi: 10.1002/path.3961. Epub 2012 Jan 27. J Pathol. 2012. PMID: 22081431 Free PMC article. - Remodeling of Murine Mammary Adipose Tissue during Pregnancy, Lactation, and Involution.
Wang QA, Scherer PE. Wang QA, et al. J Mammary Gland Biol Neoplasia. 2019 Sep;24(3):207-212. doi: 10.1007/s10911-019-09434-2. Epub 2019 Sep 12. J Mammary Gland Biol Neoplasia. 2019. PMID: 31512027 Free PMC article. Review. - A developmental atlas of rat mammary gland histology.
Masso-Welch PA, Darcy KM, Stangle-Castor NC, Ip MM. Masso-Welch PA, et al. J Mammary Gland Biol Neoplasia. 2000 Apr;5(2):165-85. doi: 10.1023/a:1026491221687. J Mammary Gland Biol Neoplasia. 2000. PMID: 11149571 Review.
Cited by
- Immune Cell Contribution to Mammary Gland Development.
Vickers R, Porter W. Vickers R, et al. J Mammary Gland Biol Neoplasia. 2024 Aug 23;29(1):16. doi: 10.1007/s10911-024-09568-y. J Mammary Gland Biol Neoplasia. 2024. PMID: 39177859 Free PMC article. Review. - An ovine model for investigation of the microenvironment of the male mammary gland.
Davies BP, Crew RC, Cochrane ALK, Davies K, Figueiredo Baptista A, Jeckel S, McCrone IS, Niu Y, Strugnell BW, Waine K, Fowden AL, Bryant CE, Wills JW, Giussani DA, Hughes K. Davies BP, et al. J Anat. 2024 Sep;245(3):405-419. doi: 10.1111/joa.14055. Epub 2024 May 12. J Anat. 2024. PMID: 38735860 Free PMC article. - The immune environment of the mammary gland fluctuates during post-lactational regression and correlates with tumour growth rate.
Hitchcock J, Hughes K, Pensa S, Lloyd-Lewis B, Watson CJ. Hitchcock J, et al. Development. 2022 Apr 15;149(8):dev200162. doi: 10.1242/dev.200162. Epub 2022 Apr 29. Development. 2022. PMID: 35420674 Free PMC article. - Macphatics and PoEMs in Postpartum Mammary Development and Tumor Progression.
Elder AM, Stoller AR, Black SA, Lyons TR. Elder AM, et al. J Mammary Gland Biol Neoplasia. 2020 Jun;25(2):103-113. doi: 10.1007/s10911-020-09451-6. Epub 2020 Jun 13. J Mammary Gland Biol Neoplasia. 2020. PMID: 32535810 Free PMC article. Review. - A Comprehensive Analysis of Runs of Homozygosity of Eleven Cattle Breeds Representing Different Production Types.
Szmatoła T, Gurgul A, Jasielczuk I, Ząbek T, Ropka-Molik K, Litwińczuk Z, Bugno-Poniewierska M. Szmatoła T, et al. Animals (Basel). 2019 Nov 25;9(12):1024. doi: 10.3390/ani9121024. Animals (Basel). 2019. PMID: 31775271 Free PMC article.
References
- Abdul Awal M, Matsumoto M, Toyoshima Y, Nishinakagawa H. Ultrastructural studies on the endothelial cells of arteries supplying the abdomino-inguinal mammary glands of rats during the reproductive cycle. J Vet Med Sci. 1996;58:29–34. - PubMed
- Andersen TI. Genetic heterogeneity in breast cancer susceptibility. Acta Oncol. 1996;35:407–410. - PubMed
- Anderson E, Clarke RB, Howell A. Changes in the normal human breast throughout the menstrual cycle: relevance to breast carcinogenesis. Endocr Relat Cancer. 1997;4:23–33.
- Andres A-C, Strange R. Apoptosis in the estrous and menstrual cycles. J Mammary Gland Biol Neoplasia. 1999;4:221–228. - PubMed
Publication types
MeSH terms
Grants and funding
- CA-77656/CA/NCI NIH HHS/United States
- P30 CA016056/CA/NCI NIH HHS/United States
- R01 CA085944/CA/NCI NIH HHS/United States
- R01 CA077656/CA/NCI NIH HHS/United States
- CA-16056/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources