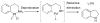Dialkylbiaryl Phosphines in Pd-Catalyzed Amination: A User's Guide - PubMed (original) (raw)
Dialkylbiaryl Phosphines in Pd-Catalyzed Amination: A User's Guide
David S Surry et al. Chem Sci. 2011.
Abstract
Dialkylbiaryl phosphines are a valuable class of ligand for Pd-catalyzed amination reactions and have been applied in a range of contexts. This review attempts to aid the reader in the selection of the best choice of reaction conditions and ligand of this class for the most commonly encountered and practically important substrate combinations.
Figures
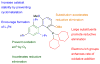
Figure 1
Important structural features of dialkylbiaryl phosphine ligands.
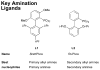
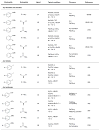
Figure 2
Key dialkylbiaryl phosphine ligands for amination.
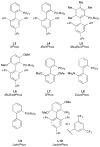
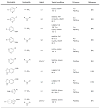
Figure 3
Other important dialkylbiaryl phosphine ligands for amination.
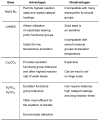
Figure 4
Comparison of bases typically used in Pd-catalyzed amination.
Figure 5
Simplistic troubleshooting guide for Pd-catalyzed amination.
Figure 6
Summary of reaction conditions used for different classes of nucleophile.
Figure 7
Summary of reaction conditions used for different classes of electrophile.
Figure 8
Summary of reaction conditions used for different classes of heteroaryl electrophile.
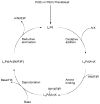
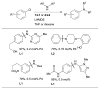
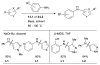
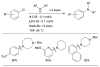
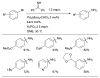
Scheme 1
Generalized catalytic cycle for Pd-catalyzed amination with dialkylbiaryl phosphines.
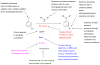
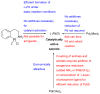
Scheme 2
Factors influencing the outcome of a Pd-catalyzed amination reaction.
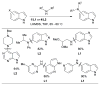
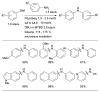
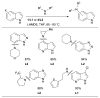
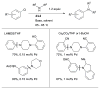
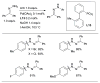
Scheme 3
Water-mediated reduction of Pd(II) salts permits efficient amination of electron-deficient anilines.
Scheme 4
Synthesis of amine bound oxidative addition precatalyst.
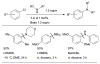
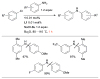
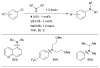
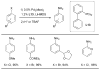
Scheme 5
Activation of intramolecularly coordinated amine oxidative addition precatalyst.
Scheme 6
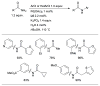
The application of precatalyst 1 allows arylation of anilines with low catalyst loading and short reaction times.
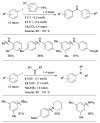
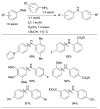
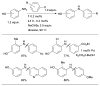
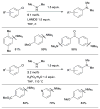
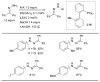
Scheme 7
Considerations for choice of Pd source for amination reactions.
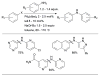
Scheme 8
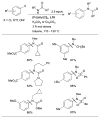
Use of LHMDS as base permits the cross-coupling of substrates bearing protic functional groups.
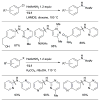
Scheme 9
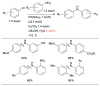
Amination of aryl chlorides at or below room temperature.
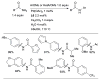
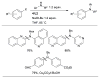
Scheme 10
Efficient Pd-catalyzed amination of aryl iodides using L1 and L2 as ligands in toluene.
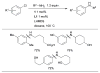
Scheme 11
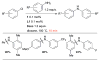
Pd-catalyzed coupling of anilines and aryl nonaflates under microwave irradiation.
Scheme 12
Pd-catalyzed coupling of anilines and aryl mesylates employing L1 as ligand.
Scheme 13
Amidation of aryl mesylates employing L6 as ligand.
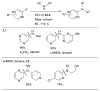
Scheme 14
Pd-catalyzed amination of 6-membered ring heteroaryl halides using L1 and L2.
Scheme 15
Amination of 5-membered ring heteroaryl halides using L1 or L2 as ligand.
Scheme 16
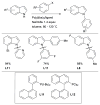
The selective arylation of 1° anilines can be conducted with high efficiency using L1.
Scheme 17
L1-based catalyst systems permit the selective N-arylation of aminophenols.
Scheme 18
L3-based catalysts allow the selective arylation of an aniline in the presence of a primary amide.
Scheme 19
L4 can be a useful ligand for the arylation of electron-deficient heteroarylamines.
Scheme 20
L1 provides an efficient catalyst system for the amination of 1° heteroarylamines under various reaction conditions.
Scheme 21
L2 is the best dialkylbiaryl phosphine for the arylation of 2° anilines.
Scheme 22
L2-based catalyst for the arylation of diarylamines.
Scheme 23
The use of an L9-based catalyst for the synthesis of triarylamines from anilines and aryl halides.
Scheme 24
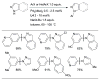
L1 permits the coupling of 1° aliphatic amines with low catalyst loadings and short reaction times.
Scheme 25
L1 is the best ligand for the reaction of 1° aliphatic amines.
Scheme 26
L1-based catalyst systems provide excellent selectivity for the arylation of 1° amines in the presence of 2° amines.
Scheme 27
The efficient monoarylation of methylamine can be accomplished by the use of a L1-based catalyst system.
Scheme 28
L2 can be used for the arylation of cyclic 2° aliphatic amines under a variety of conditions.
Scheme 29
L2 can be used for the arylation of cyclic 2° aliphatic amines under a variety of conditions.
Scheme 30
L2 is the most effective ligand for the cross-coupling of acyclic aliphatic amines.
Scheme 31
Catalysts based on L3 or L4 can affect the arylation of dimethylamine.
Scheme 32
L6 is the best dialkylbiaryl phosphine for the arylation of 1° amides.
Scheme 33
Arylation of 2° amides, ureas, carbamates and sulfonamides is possible by using L10 as ligand.
Scheme 34
A variety of dialkylbiaryl phosphine ligands are suitable for the N-arylation of indoles.
Scheme 35
L4 is a useful ligand for the arylation of indazoles and pyrrazoles.
Scheme 36
The arylation of imidazole and benzimidazole can be brought about in some cases by using L5 as ligand.
Scheme 37
L4 is a useful ligand for the conversion of aryl bromides to anilines.
Scheme 38
L13 is a useful ligand for the conversion of aryl halides to anilines using LiHMDS as the ammonia surrogate.
Scheme 39
Benzophenone hydrazone can be effectively arylated with aryl chlorides and bromides.
Similar articles
- Rationale behind the resistance of dialkylbiaryl phosphines toward oxidation by molecular oxygen.
Barder TE, Buchwald SL. Barder TE, et al. J Am Chem Soc. 2007 Apr 25;129(16):5096-101. doi: 10.1021/ja0683180. Epub 2007 Mar 28. J Am Chem Soc. 2007. PMID: 17388595 - Development of an Aryl Amination Catalyst with Broad Scope Guided by Consideration of Catalyst Stability.
McCann SD, Reichert EC, Arrechea PL, Buchwald SL. McCann SD, et al. J Am Chem Soc. 2020 Sep 2;142(35):15027-15037. doi: 10.1021/jacs.0c06139. Epub 2020 Aug 24. J Am Chem Soc. 2020. PMID: 32786769 Free PMC article. - Ylide-Substituted Phosphines: A Platform of Strong Donor Ligands for Gold Catalysis and Palladium-Catalyzed Coupling Reactions.
Lapointe S, Sarbajna A, Gessner VH. Lapointe S, et al. Acc Chem Res. 2022 Mar 1;55(5):770-782. doi: 10.1021/acs.accounts.1c00797. Epub 2022 Feb 16. Acc Chem Res. 2022. PMID: 35170935 - Palladium N-Heterocyclic Carbene-Catalyzed Aminations: An Outline.
Umabharathi SB, Neetha M, Anilkumar G. Umabharathi SB, et al. Top Curr Chem (Cham). 2024 Jan 24;382(1):3. doi: 10.1007/s41061-024-00449-w. Top Curr Chem (Cham). 2024. PMID: 38265533 Review. - Advances in transition metal-catalyzed C-H amination strategies using anthranils.
Aher YN, Bhaduri N, Pawar AB. Aher YN, et al. Org Biomol Chem. 2023 Nov 15;21(44):8794-8812. doi: 10.1039/d3ob01421e. Org Biomol Chem. 2023. PMID: 37901918 Review.
Cited by
- Total Synthesis of (-)-Nodulisporic Acid D.
Zou Y, Melvin JE, Gonzales SS, Spafford MJ, Smith AB 3rd. Zou Y, et al. J Am Chem Soc. 2015 Jun 10;137(22):7095-8. doi: 10.1021/jacs.5b04728. Epub 2015 Jun 1. J Am Chem Soc. 2015. PMID: 26029849 Free PMC article. - Photoredox Catalysis in Organic Chemistry.
Shaw MH, Twilton J, MacMillan DW. Shaw MH, et al. J Org Chem. 2016 Aug 19;81(16):6898-926. doi: 10.1021/acs.joc.6b01449. Epub 2016 Aug 1. J Org Chem. 2016. PMID: 27477076 Free PMC article. - Cu(i)-catalyzed cross-coupling of primary amines with 2,2'-dibromo-1,1'-biphenyl for the synthesis of polysubstituted carbazole.
Niu YN, Qiao Y, Wang KY, Sha BX, Li GQ. Niu YN, et al. RSC Adv. 2022 Aug 25;12(37):24232-24236. doi: 10.1039/d2ra03323b. eCollection 2022 Aug 22. RSC Adv. 2022. PMID: 36128530 Free PMC article. - Structure-Based Design of Tetrahydroisoquinoline-7-carboxamides as Selective Discoidin Domain Receptor 1 (DDR1) Inhibitors.
Wang Z, Bian H, Bartual SG, Du W, Luo J, Zhao H, Zhang S, Mo C, Zhou Y, Xu Y, Tu Z, Ren X, Lu X, Brekken RA, Yao L, Bullock AN, Su J, Ding K. Wang Z, et al. J Med Chem. 2016 Jun 23;59(12):5911-6. doi: 10.1021/acs.jmedchem.6b00140. Epub 2016 Jun 3. J Med Chem. 2016. PMID: 27219676 Free PMC article. - From Quinoxaline, Pyrido[2,3-b]pyrazine and Pyrido[3,4-b]pyrazine to Pyrazino-Fused Carbazoles and Carbolines.
Lassagne F, Langlais T, Caytan E, Limanton E, Paquin L, Boullard M, Courtel C, Curbet I, Gédéon C, Lebreton J, Picot L, Thiéry V, Souab M, Baratte B, Ruchaud S, Bach S, Roisnel T, Mongin F. Lassagne F, et al. Molecules. 2018 Nov 13;23(11):2961. doi: 10.3390/molecules23112961. Molecules. 2018. PMID: 30428591 Free PMC article.
References
- King AO, Yasuda N. Topics in Organometallic Chemistry. 2004;6:205–245.
- Torborg C, Beller M. Adv Synth Catal. 2009;351:3027–3043.
- Carey JS, Laffan D, Thomson C, Williams MT. Org Biomol Chem. 2006;4:2337–2347. - PubMed
- Baeza A, Burgos C, Alvarez-Builla J, Vaquero JJ. Tetrahedron Lett. 2007;48:2597–2601.
LinkOut - more resources
Full Text Sources
Other Literature Sources