The noncanonical NF-κB pathway - PubMed (original) (raw)
Review
The noncanonical NF-κB pathway
Shao-Cong Sun. Immunol Rev. 2012 Mar.
Abstract
The noncanonical nuclear factor-κB (NF-κB) signaling pathway mediates activation of the p52/RelB NF-κB complex and, thereby, regulates specific immunological processes. This NF-κB pathway relies on the inducible processing of NF-κB2 precursor protein, p100, as opposed to the degradation of IκBα in the canonical NF-κB pathway. A central signaling component of the noncanonical NF-κB pathway is NF-κB-inducing kinase (NIK), which functions together with a downstream kinase, IKKα (inhibitor of NF-κB kinase α), to induce phosphorylation-dependent ubiquitination and processing of p100. Under normal conditions, NIK is targeted for continuous degradation by a tumor necrosis factor (TNF) receptor-associated factor-3 (TRAF3)-dependent E3 ubiquitin ligase. In response to signals mediated by a subset of TNF receptor superfamily members, NIK becomes stabilized as a result of TRAF3 degradation, leading to the activation of noncanonical NF-κB. This review discusses both the historical perspectives and the recent progress in the regulation and biological function of the noncanonical NF-κB pathway.
© 2012 John Wiley & Sons A/S.
Conflict of interest statement
The author has no conflicts of interest to declare.
Figures
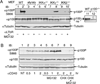
Fig. 1. In vivo phosphorylation of p100
(A) The indicated MEF cells were either not treated (−) or stimulated for 2 h with anti-murine LTβR (1 µg/ml) together with the proteasome inhibitor MG132 (25 µM). IB assays were performed to detect the phosphorylated p100 (p100P) and total p100 (p100) as well as the loading control tubulin using the indicated antibodies. Lane 11 and 12 show the cross-reactivity of the anti-p100 antibody with a non-specific protein (ns), as demonstrated by using the nfκb2 KO (p100−/−) MEFs. (B) M12 B cells expressing human CD40 (M12-CD40) were either not treated (NT) or stimulated for the indicated times with anti-human CD40. Where indicated, the cells were stimulated in the presence of MG132 and/or cycloheximide (CHX, 5 µg/ml). IB was performed using the indicated antibodies. This figure is adapted from Liang et al. (16) with permission.
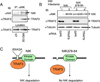
Fig. 2. NIK degradation through NIK-TRAF3 association
(A) M12 B cells were either not treated (NT) or incubated for 2 h with proteasome inhibitor MG132. The NIK protein complex was isolated by IP followed by detection of NIK (top panel) and TRAF3 (middle panel) by IB using anti-NIK and horseradish peroxidase-conjugated anti-TRAF3, respectively. The TRAF3 expression level was detected by direct IB (bottom level). (B) 293 cells were infected with retroviruses encoding NIK or a NIK mutant lacking the core region of TRAF3-binding site (NIKΔ78–84). Stably infected cells were transfected with small-interfering RNA (siRNA) for either the control GFP or TRAF3. Cell lysates were subjected to IB using the indicated antibodies to detect the expression level of NIK (top panel), TRAF3 (panel 2), or control tubulin (panel 3). (C) Schematic picture depicting the regulation of NIK degradation by TRAF3. Wildtype NIK is bound by TRAF3 via an N-terminal domain and targeted for continuous degradation, which explains why NIK is barely detectable unless when the cells are treated with a proteasome inhibitor (A) or TRAF3 siRNA (B). NIKΔ78–84 is not bound by TRAF3 and stable even in the presence of TRAF3. This figure is adapted from Liao et al. (29) with permission.
Fig. 3. Signal-induced NIK activation involves TRAF3 degradation and NIK accumulation
M12 cells and M12-NIK cells (M12 cells stably infected with retroviruses encoding NIK) were either not treated (NT) or incubated for 2 h with anti-CD40, BAFF, or TNF-α. Whole-cell lysates were subjected to IP using anti-NIK (lanes 1–7 and 9) or a preimmune serum (lane 8) followed by detection of the enriched NIK protein by IB (top panel). The expression of TRAF3 (middle panel) and control tubulin (bottom panel) was detected by direct IB. This figure is adapted from Liao et al. (29) with permission.
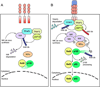
Fig. 4. A model of noncanonical NF-κB regulation
(A) Under normal conditions, de novo synthesized NIK is immediately bound by TRAF3 and recruited to the TRAF-cIAP E3 ubiquitin ligase complex via TRAF3-TRAF2 dimerization. cIAP1/2 catalyzes K48 ubiquitination of NIK, targeting NIK for degradation in the proteasome. The continuous degradation of NIK prevents NIK accumulation or activation of noncanonical NF-κB signaling. (B) Ligand engagement of the noncanonical NF-κB-stimulatory receptors induces recruitment of the TRAF-cIAP E3 components to the receptor complex. Probably due to its aggregation, TRAF2 becomes activated and mediates K63 ubiquitination of cIAP1/2, a modification that stimulates the K48-specific E3 activity of cIAP1/2. cIAP1/2 then conjugates K48 polyubiquitin chains to TRAF3, resulting in TRAF3 degradation. In the absence of TRAF3, the de novo synthesized NIK is stabilized and accumulated to higher levels; this allows NIK to induce IKKα-dependent p100 phosphorylation and ubiquitination, leading to the nuclear translocation of the generated RelB/p52 heterodimer.
Similar articles
- The NF-κB subunit RelB controls p100 processing by competing with the kinases NIK and IKK1 for binding to p100.
Fusco AJ, Mazumder A, Wang VY, Tao Z, Ware C, Ghosh G. Fusco AJ, et al. Sci Signal. 2016 Sep 27;9(447):ra96. doi: 10.1126/scisignal.aad9413. Sci Signal. 2016. PMID: 27678221 - Maximal adamantyl-substituted retinoid-related molecule-induced apoptosis requires NF-κB noncanonical and canonical pathway activation.
Farhana L, Dawson MI, Murshed F, Fontana JA. Farhana L, et al. Cell Death Differ. 2011 Jan;18(1):164-73. doi: 10.1038/cdd.2010.84. Epub 2010 Jul 30. Cell Death Differ. 2011. PMID: 20671747 Free PMC article. - Non-canonical NF-κB signaling pathway.
Sun SC. Sun SC. Cell Res. 2011 Jan;21(1):71-85. doi: 10.1038/cr.2010.177. Epub 2010 Dec 21. Cell Res. 2011. PMID: 21173796 Free PMC article. Review. - Negative feedback in noncanonical NF-kappaB signaling modulates NIK stability through IKKalpha-mediated phosphorylation.
Razani B, Zarnegar B, Ytterberg AJ, Shiba T, Dempsey PW, Ware CF, Loo JA, Cheng G. Razani B, et al. Sci Signal. 2010 May 25;3(123):ra41. doi: 10.1126/scisignal.2000778. Sci Signal. 2010. PMID: 20501937 Free PMC article. - Targeting signaling factors for degradation, an emerging mechanism for TRAF functions.
Yang XD, Sun SC. Yang XD, et al. Immunol Rev. 2015 Jul;266(1):56-71. doi: 10.1111/imr.12311. Immunol Rev. 2015. PMID: 26085207 Free PMC article. Review.
Cited by
- Mitochondrial Contribution to Inflammation in Diabetic Kidney Disease.
Mitrofanova A, Fontanella AM, Burke GW, Merscher S, Fornoni A. Mitrofanova A, et al. Cells. 2022 Nov 16;11(22):3635. doi: 10.3390/cells11223635. Cells. 2022. PMID: 36429063 Free PMC article. Review. - Evaluating the Potential of Plukenetia volubilis Linneo (Sacha Inchi) in Alleviating Cardiovascular Disease Risk Factors: A Mini Review.
Abd Rahman IZ, Nor Hisam NS, Aminuddin A, Hamid AA, Kumar J, Ugusman A. Abd Rahman IZ, et al. Pharmaceuticals (Basel). 2023 Nov 9;16(11):1588. doi: 10.3390/ph16111588. Pharmaceuticals (Basel). 2023. PMID: 38004453 Free PMC article. Review. - Inhibition of inflammatory arthritis using fullerene nanomaterials.
Dellinger AL, Cunin P, Lee D, Kung AL, Brooks DB, Zhou Z, Nigrovic PA, Kepley CL. Dellinger AL, et al. PLoS One. 2015 Apr 16;10(4):e0126290. doi: 10.1371/journal.pone.0126290. eCollection 2015. PLoS One. 2015. PMID: 25879437 Free PMC article. - Mitogen-Activated Protein Kinase 14 Promotes AKI.
Ortiz A, Husi H, Gonzalez-Lafuente L, Valiño-Rivas L, Fresno M, Sanz AB, Mullen W, Albalat A, Mezzano S, Vlahou T, Mischak H, Sanchez-Niño MD. Ortiz A, et al. J Am Soc Nephrol. 2017 Mar;28(3):823-836. doi: 10.1681/ASN.2015080898. Epub 2016 Sep 12. J Am Soc Nephrol. 2017. PMID: 27620989 Free PMC article. - TNFR2 ligation in human T regulatory cells enhances IL2-induced cell proliferation through the non-canonical NF-κB pathway.
Wang J, Ferreira R, Lu W, Farrow S, Downes K, Jermutus L, Minter R, Al-Lamki RS, Pober JS, Bradley JR. Wang J, et al. Sci Rep. 2018 Aug 13;8(1):12079. doi: 10.1038/s41598-018-30621-4. Sci Rep. 2018. PMID: 30104686 Free PMC article.
References
- Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. - PubMed
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI064639/AI/NIAID NIH HHS/United States
- AI064639/AI/NIAID NIH HHS/United States
- R01 AI057555/AI/NIAID NIH HHS/United States
- GM84459/GM/NIGMS NIH HHS/United States
- R37 AI064639/AI/NIAID NIH HHS/United States
- GM84459-S1/GM/NIGMS NIH HHS/United States
- AI057555/AI/NIAID NIH HHS/United States
- R01 GM084459/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous