Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference - PubMed (original) (raw)
Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference
Luke J Chang et al. Cereb Cortex. 2013 Mar.
Abstract
Recent work has indicated that the insula may be involved in goal-directed cognition, switching between networks, and the conscious awareness of affect and somatosensation. However, these findings have been limited by the insula's remarkably high base rate of activation and considerable functional heterogeneity. The present study used a relatively unbiased data-driven approach combining resting-state connectivity-based parcellation of the insula with large-scale meta-analysis to understand how the insula is anatomically organized based on functional connectivity patterns as well as the consistency and specificity of the associated cognitive functions. Our findings support a tripartite subdivision of the insula and reveal that the patterns of functional connectivity in the resting-state analysis appear to be relatively conserved across tasks in the meta-analytic coactivation analysis. The function of the networks was meta-analytically "decoded" using the Neurosynth framework and revealed that while the dorsoanterior insula is more consistently involved in human cognition than ventroanterior and posterior networks, each parcellated network is specifically associated with a distinct function. Collectively, this work suggests that the insula is instrumental in integrating disparate functional systems involved in processing affect, sensory-motor processing, and general cognition and is well suited to provide an interface between feelings, cognition, and action.
Figures
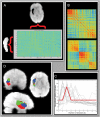
Figure 1.
Functional parcellation method. Panel A depicts a high-resolution right insula (2.5 × 2.5 × 3 mm3) by low-resolution rest of brain matrix (5 × 5 × 6 mm3) of voxel-wise time series correlations. Panel B depicts 2 correlation matrices of voxels in the insula based on their pattern of connectivity with the rest of the brain. The upper matrix is the unordered matrix for one subject. The lower matrix is the same matrix ordered by the clustering algorithm. Panel C depicts the VI metric, which selects 3 as the optimal number of clusters (k). Panel D depicts the 3D spatial maps sorted by the results of the cluster analysis.
Figure 2.
Results of the functional parcellation analysis. Figure 2 depicts the number of subjects loading on each cluster for each voxel in the insula.
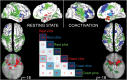
Figure 3.
Positively connected functionally parcellated networks. Figure 3 depicts the brain networks that are functionally coupled to each insular subregion controlling for activity in other subregions. The resting-state analysis assesses functional connectivity using multilevel multiple regression. The coactivation analysis highlights networks that are coactive across studies in the Neurosynth database using multiple logistic regression. vIns (red) = networks connected to the ventroanterior region of the insula. dIns (blue) = networks connected to the dorsoanterior region of the insula. pIns (green) = networks connected to the posterior insular region. Images are presented using neurological conventions (i.e., right = right). Both analyses are thresholded using whole brain cluster correction with an initial cluster threshold of Z > 2.3 for the resting state and Z > 4.5 for the coactivation and a Family Wise Error corrected threshold of P < 0.05. The correlation matrix reflects the spatial coherence of the networks using Pearson correlations multiplied by 100.
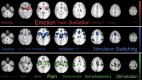
Figure 4.
Functional rankings of coactivation maps. Figure 4 depicts the results of the decoding analysis. The top 15 topics from the reverse inference analysis that were associated with each Meta-Analytic Functional Coactivation Analysis network map. Red = network associated with ventroanterior insula. Blue = network coupled with dorsoanterior insula. Green = network connected to posterior insula. Images are presented in neurological orientation and thresholded using whole brain cluster correction with an initial cluster threshold of Z > 4.5 and a Family Wise Error corrected threshold of P < 0.05 size and transparency of word clouds reflect rank-ordered correlation coefficients exponentiated to the 2nd power, which emphasizes terms that are most associated with each network (Poldrack et al. 2009).
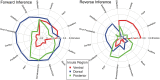
Figure 5.
Consistency and specificity of functions associated with meta-analytic coactivation of parcellated insular networks. The correlation values for the top 5 topics for each cluster using forward and reverse inference. Topics were selected if they were present in both forward and reverse inference analyses and represented nonmethod terms.
Comment in
- How many clusters in the insular cortex?
Cauda F, Vercelli A. Cauda F, et al. Cereb Cortex. 2013 Nov;23(11):2779-80. doi: 10.1093/cercor/bhs249. Epub 2012 Aug 14. Cereb Cortex. 2013. PMID: 22892421 No abstract available.
Similar articles
- Altered functional organization within the insular cortex in adult males with high-functioning autism spectrum disorder: evidence from connectivity-based parcellation.
Yamada T, Itahashi T, Nakamura M, Watanabe H, Kuroda M, Ohta H, Kanai C, Kato N, Hashimoto RI. Yamada T, et al. Mol Autism. 2016 Oct 5;7:41. doi: 10.1186/s13229-016-0106-8. eCollection 2016. Mol Autism. 2016. PMID: 27713815 Free PMC article. - Functional Segregation of the Human Dorsomedial Prefrontal Cortex.
Eickhoff SB, Laird AR, Fox PT, Bzdok D, Hensel L. Eickhoff SB, et al. Cereb Cortex. 2016 Jan;26(1):304-21. doi: 10.1093/cercor/bhu250. Epub 2014 Oct 20. Cereb Cortex. 2016. PMID: 25331597 Free PMC article. - Comparison of structural covariance with functional connectivity approaches exemplified by an investigation of the left anterior insula.
Clos M, Rottschy C, Laird AR, Fox PT, Eickhoff SB. Clos M, et al. Neuroimage. 2014 Oct 1;99:269-80. doi: 10.1016/j.neuroimage.2014.05.030. Epub 2014 May 17. Neuroimage. 2014. PMID: 24844743 Free PMC article. - The Insula: A "Hub of Activity" in Migraine.
Borsook D, Veggeberg R, Erpelding N, Borra R, Linnman C, Burstein R, Becerra L. Borsook D, et al. Neuroscientist. 2016 Dec;22(6):632-652. doi: 10.1177/1073858415601369. Epub 2015 Aug 19. Neuroscientist. 2016. PMID: 26290446 Free PMC article. Review. - Large-Scale Meta-Analysis of Human Medial Frontal Cortex Reveals Tripartite Functional Organization.
de la Vega A, Chang LJ, Banich MT, Wager TD, Yarkoni T. de la Vega A, et al. J Neurosci. 2016 Jun 15;36(24):6553-62. doi: 10.1523/JNEUROSCI.4402-15.2016. J Neurosci. 2016. PMID: 27307242 Free PMC article. Review.
Cited by
- Neuroimaging-based biomarkers for treatment selection in major depressive disorder.
Dunlop BW, Mayberg HS. Dunlop BW, et al. Dialogues Clin Neurosci. 2014 Dec;16(4):479-90. doi: 10.31887/DCNS.2014.16.4/bdunlop. Dialogues Clin Neurosci. 2014. PMID: 25733953 Free PMC article. Review. - Cognitive Vulnerability to Major Depression: View from the Intrinsic Network and Cross-network Interactions.
Wang X, Öngür D, Auerbach RP, Yao S. Wang X, et al. Harv Rev Psychiatry. 2016 May-Jun;24(3):188-201. doi: 10.1097/HRP.0000000000000081. Harv Rev Psychiatry. 2016. PMID: 27148911 Free PMC article. Review. - Alterations of Heartbeat Evoked Magnetic Fields Induced by Sounds of Disgust.
Kato Y, Takei Y, Umeda S, Mimura M, Fukuda M. Kato Y, et al. Front Psychiatry. 2020 Jul 22;11:683. doi: 10.3389/fpsyt.2020.00683. eCollection 2020. Front Psychiatry. 2020. PMID: 32792994 Free PMC article. - Resting-State Functional Connectivity Profile of Insular Subregions.
Ghaziri J, Fei P, Tucholka A, Obaid S, Boucher O, Rouleau I, Nguyen DK. Ghaziri J, et al. Brain Sci. 2024 Jul 25;14(8):742. doi: 10.3390/brainsci14080742. Brain Sci. 2024. PMID: 39199437 Free PMC article. - Increased Functional Connectivity in an Insula-Based Network is Associated with Improved Smoking Cessation Outcomes.
Addicott MA, Sweitzer MM, Froeliger B, Rose JE, McClernon FJ. Addicott MA, et al. Neuropsychopharmacology. 2015 Oct;40(11):2648-56. doi: 10.1038/npp.2015.114. Epub 2015 Apr 21. Neuropsychopharmacology. 2015. PMID: 25895453 Free PMC article.
References
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–244. - PubMed
- Bamiou DE, Musiek FE, Luxon LM. The insula (Island of Reil) and its role in auditory processing. Literature review. Brain Res Brain Res Rev. 2003;42:143–154. - PubMed
- Blei D, Ng A, Jordan M. Latent Dirichlet allocation. J Mach Learn Res. 2003;3:993–1022.