Targeting natural killer cells and natural killer T cells in cancer - PubMed (original) (raw)
Review
Targeting natural killer cells and natural killer T cells in cancer
Eric Vivier et al. Nat Rev Immunol. 2012.
Abstract
Natural killer (NK) cells and natural killer T (NKT) cells are subsets of lymphocytes that share some phenotypical and functional similarities. Both cell types can rapidly respond to the presence of tumour cells and participate in antitumour immune responses. This has prompted interest in the development of innovative cancer therapies that are based on the manipulation of NK and NKT cells. Recent studies have highlighted how the immune reactivity of NK and NKT cells is shaped by the environment in which they develop. The rational use of these cells in cancer immunotherapies awaits a better understanding of their effector functions, migratory patterns and survival properties in humans.
Figures
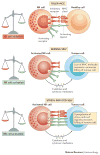
Figure 1
NK cell recognition of tumour cells. a | NK cells are tolerant to normal self cells as the ‘strength’ of activating signals is dampened by engagement of inhibitory receptors. b | NK cells are selectively activated by stressed cells as they express a density of cell surface ligands for activating receptors which overcomes signalling via inhibitory receptors. c | this NK cell activation leads to tumour elimination directly (cytotoxicity) or indirectly (production of cytokines such as IFN-γ).
Figure 2
NK cellular therapy. a | In haplo-identical or MHC-matched HSCT, NK cells of healthy donor origin will develop into the cancer patient. b | Alternatively, NK cells can be isolated from healthy donors, activated and/or expanded in vitro prior to infusion into the cancer patient. In both cases (allo-HSCT and NK cell infusions), the aim is to promote the anti-tumour function of donor NK cells in the cancer patient; indeed a fraction of donor NK cells will be not be inhibited by the MHC class I molecules of the cancer patient as the KIR expressed by these subsets of NK cells will not interact with by the MHC class I molecules of the cancer patient (ELIMINATION). Most normal cells of the patients will not activate donor NK cells as, in contrast to cancer cells of the patient, they lack the cell surface density of ligands required to activate donor NK cells (IGNORANCE). c | Anti-KIR monoclonal antibody therapy. Fully human anti-KIR mAbs can be injected in cancer patient. Anti-KIR mAbs are designed to boost the antitumour activity of NK cells (ELIMINATION) without inducing auto-immunity (IGNORANCE).
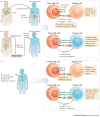
Figure 3
iNKT cell recognition of tumours. a | normal and direct recognition of tumour cells by iNKT cells. b | Indirect activation and indirect killing mediated by iNKT cells. c | Control of angiogenesis by iNKT cells
Figure 4
iNKT cellular therapy. Patient Specific iNKT cell subset expanded in vitro prior to infusion together with CD1d ligand pulsed Dendritic cells.
Similar articles
- The Role of Natural Killer T Cells in Cancer-A Phenotypical and Functional Approach.
Krijgsman D, Hokland M, Kuppen PJK. Krijgsman D, et al. Front Immunol. 2018 Feb 27;9:367. doi: 10.3389/fimmu.2018.00367. eCollection 2018. Front Immunol. 2018. PMID: 29535734 Free PMC article. Review. - Characterization of circulating T-, NK-, and NKT cell subsets in patients with colorectal cancer: the peripheral blood immune cell profile.
Krijgsman D, de Vries NL, Skovbo A, Andersen MN, Swets M, Bastiaannet E, Vahrmeijer AL, van de Velde CJH, Heemskerk MHM, Hokland M, Kuppen PJK. Krijgsman D, et al. Cancer Immunol Immunother. 2019 Jun;68(6):1011-1024. doi: 10.1007/s00262-019-02343-7. Epub 2019 May 3. Cancer Immunol Immunother. 2019. PMID: 31053876 Free PMC article. - Possible Therapeutic Application of Targeting Type II Natural Killer T Cell-Mediated Suppression of Tumor Immunity.
Kato S, Berzofsky JA, Terabe M. Kato S, et al. Front Immunol. 2018 Feb 22;9:314. doi: 10.3389/fimmu.2018.00314. eCollection 2018. Front Immunol. 2018. PMID: 29520281 Free PMC article. Review. - Chimeric antigen receptor-engineered natural killer and natural killer T cells for cancer immunotherapy.
Bollino D, Webb TJ. Bollino D, et al. Transl Res. 2017 Sep;187:32-43. doi: 10.1016/j.trsl.2017.06.003. Epub 2017 Jun 9. Transl Res. 2017. PMID: 28651074 Free PMC article. Review. - Characterization of natural killer and natural killer-like T cells derived from ex vivo expanded and activated cord blood mononuclear cells: implications for adoptive cellular immunotherapy.
Ayello J, van de Ven C, Cairo E, Hochberg J, Baxi L, Satwani P, Cairo MS. Ayello J, et al. Exp Hematol. 2009 Oct;37(10):1216-29. doi: 10.1016/j.exphem.2009.07.009. Epub 2009 Jul 26. Exp Hematol. 2009. PMID: 19638292
Cited by
- Molecular signatures mostly associated with NK cells are predictive of relapse free survival in breast cancer patients.
Ascierto ML, Idowu MO, Zhao Y, Khalak H, Payne KK, Wang XY, Dumur CI, Bedognetti D, Tomei S, Ascierto PA, Shanker A, Bear HD, Wang E, Marincola FM, De Maria A, Manjili MH. Ascierto ML, et al. J Transl Med. 2013 Jun 12;11:145. doi: 10.1186/1479-5876-11-145. J Transl Med. 2013. PMID: 23758773 Free PMC article. - Human hemato-lymphoid system mice: current use and future potential for medicine.
Rongvaux A, Takizawa H, Strowig T, Willinger T, Eynon EE, Flavell RA, Manz MG. Rongvaux A, et al. Annu Rev Immunol. 2013;31:635-674. doi: 10.1146/annurev-immunol-032712-095921. Epub 2013 Jan 16. Annu Rev Immunol. 2013. PMID: 23330956 Free PMC article. Review. - Modulating barriers of tumor microenvironment through nanocarrier systems for improved cancer immunotherapy: a review of current status and future perspective.
Lan H, Zhang W, Jin K, Liu Y, Wang Z. Lan H, et al. Drug Deliv. 2020 Dec;27(1):1248-1262. doi: 10.1080/10717544.2020.1809559. Drug Deliv. 2020. PMID: 32865029 Free PMC article. Review. - NK cells: key to success of DC-based cancer vaccines?
Lion E, Smits EL, Berneman ZN, Van Tendeloo VF. Lion E, et al. Oncologist. 2012;17(10):1256-70. doi: 10.1634/theoncologist.2011-0122. Epub 2012 Aug 20. Oncologist. 2012. PMID: 22907975 Free PMC article. Review. - Evaluation of natural killer cell (CD57) as a prognostic marker in oral squamous cell carcinoma: An immunohistochemistry study.
Agarwal R, Chaudhary M, Bohra S, Bajaj S. Agarwal R, et al. J Oral Maxillofac Pathol. 2016 May-Aug;20(2):173-7. doi: 10.4103/0973-029X.185933. J Oral Maxillofac Pathol. 2016. PMID: 27601804 Free PMC article.
References
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. - PubMed
- Beutler B. Innate immunity: an overview. Mol Immunol. 2004;40:845–59. - PubMed
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. - PubMed
- Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–7. - PubMed
Publication types
MeSH terms
Grants and funding
- AI058181/AI/NIAID NIH HHS/United States
- R01 AI058181/AI/NIAID NIH HHS/United States
- AI46709/AI/NIAID NIH HHS/United States
- R01 AI046709/AI/NIAID NIH HHS/United States
- R56 AI058181/AI/NIAID NIH HHS/United States
- R21 AI046709/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical